��Ŀ����
��1����ͼ��������������������֤��е���غ㶨�ɵ�ʵ��װ�ã�����m��1.00kg�������������䣬����ʱ����ֽ���ϴ��һϵ�е㣮��ͼ��ʾΪѡȡ��һ������ʵ��Ҫ���ֽ����O Ϊ��һ���㣬A��B��C Ϊ�Ӻ���λ�ÿ�ʼѡȡ������������(������δ����)����֪����ʱ��ÿ�� 0.02s��һ�ε㣬���ص��������ٶ�g��9.80m/s2
��ijλͬѧ������֤��е���غ㶨�ɡ���ʵ�飬���в��������д������(���� )
A���ѵ�Ŵ���ʱ���̶�������̨�ϣ��õ������ӵ���ѹ������Դ
B���������ش���ֽ��������λ�ף���ֽ�����ش�������һ���߶�
C�����ͷ�ֽ�����ٽ�ͨ��Դ
D������ֽ�����ظ�ʵ�飬���ݼ�¼��������
��ѡȡO �㵽B������֤��е���غ㶨�ɣ���������������ܼ�����Ϊ ��________ J�����ܵ�������Ϊ
��________ J�����ܵ�������Ϊ ��_______ J��(���ȡ��λ��Ч����) ʵ��Ľ����� ��
��_______ J��(���ȡ��λ��Ч����) ʵ��Ľ����� ��
��2����10�֣�ijС�����ϱ��С�6V 3W����������Ҫ��������������ʱ��ʵ�ʵ��裬ʵ�����ṩ�������ģ�
A����ѹ�����̣�0��3V������Լ2k����
B����ѹ�����̣�0��l5V������Լ6k����
C�����������̣�0��0.6A������Լ0.5����
D�����������̣�0��3A������Լ0.1����
E������������ (20����2A)
F��ѧ����Դ(ֱ��10V)�����ء����ߵ�
��ʵ���е�ѹ������Ӧѡ��_______���A����B����������������Ӧѡ��_______���C����D���� ��
�ڰ���ʵ���·ͼ��ͼ12���а�ʵ���ʵ������ͼ����������

��ȷ���ӵ�·���ڱպϿ���ǰ�����������Ļ�ƬӦ���Ƶ�_______�ˣ���������ҡ�����
�ܱպϿ��غ��ڻ���������ʹС���ݴﵽ���������������ѹ����ʱ������ʾ����ͼ12����ʾ����С���ݵĵ���IJ���ֵΪ_______��
��1) �� C �� ��
��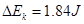 ������������ķ�Χ�ڻ�е���غ㡣
������������ķ�Χ�ڻ�е���غ㡣
��2���� B C ����ʵ��ͼ��������ͼ
������ �� �� 12.5����
���������������1����C�������д���Ӧ�Ƚ�ͨ��Դ��Ȼ���ͷ��������ֽ����ʼ���ǿհף��������ɼ������ݲ����������˷�ֽ�����ڴ�O��B�ľ��룺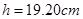 ����
����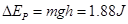 ��B���ٶ�Ϊ
��B���ٶ�Ϊ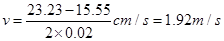 ��
�� �������ݶԱȿɵã�
�������ݶԱȿɵã� �Դ���
�Դ��� �����ڸ�ʵ����ڿ���������ֽ��������֮���Ħ��������������أ��ʽ����ǣ�����������ķ�Χ�ڣ���������������е���غ㶨�ɡ�
�����ڸ�ʵ����ڿ���������ֽ��������֮���Ħ��������������أ��ʽ����ǣ�����������ķ�Χ�ڣ���������������е���غ㶨�ɡ�
��2���ٵ��ݵĶ��ѹʱ6V����ѡ������Ϊ15V�ĵ�ѹ��B������Ϊ3V�ĵ�ѹ���ڵ�����������ʱ�ᳬ��������̣���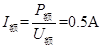 ����ѡ������Ϊ0.6�ĵ�����C��3A�ĵ�������ʹ������С������ �ڵ�·ͼʹ�õ�����ӷ�+����ʽ��·����裬����ʵ������ͼ��ʾ����Ϊ��ʹ�����������Ե�·�𱣻����ã�����������Ҫ�ֵ�����ĵ�ѹ������������ʹ������ʽ�ӷ�����߲��ִ����ڵ�·�У��ڱպϿ���ǰ��Ӧ����Ƭ�Ƶ���ʹ�����·����ֵ��������Ҷˡ��ܸ���ͼ12�Ҷ�������
����ѡ������Ϊ0.6�ĵ�����C��3A�ĵ�������ʹ������С������ �ڵ�·ͼʹ�õ�����ӷ�+����ʽ��·����裬����ʵ������ͼ��ʾ����Ϊ��ʹ�����������Ե�·�𱣻����ã�����������Ҫ�ֵ�����ĵ�ѹ������������ʹ������ʽ�ӷ�����߲��ִ����ڵ�·�У��ڱպϿ���ǰ��Ӧ����Ƭ�Ƶ���ʹ�����·����ֵ��������Ҷˡ��ܸ���ͼ12�Ҷ�������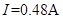 ����
���� ��
��
���㣺���⿼������֤��е���غ㶨�ɡ�����������衣

��ͼ����ʾ��ijͬѧ̽�����ٶ������Ĺ�ϵ��ʵ��װ�ã��������浼���ϰ�װ��һ�������B�������Ϲ̶�һ�ڹ�����������ϸ���ƹ����浼����˵Ķ����������������������������·����ҹ��룬ÿ�λ��鶼��A���ɾ�ֹ�ͷţ�

�ٸ�ͬѧ���α꿨�߲����ڹ����Ŀ���d����ͼ����ʾ����d= mm��
��ʵ��ʱ���������Aλ���ɾ�ֹ�ͷţ������ּ�ʱ�������ڹ���ͨ�������B��ʱ��t����Ҫ�õ�����ļ��ٶȣ�����Ҫ�������������� ��
�����в���Ҫ��һ��ʵ��Ҫ���� ��������дѡ��ǰ��Ӧ����ĸ��
| A��Ӧʹ��������Զ���ڹ�������������������� |
| B��ӦʹAλ�������ż�ľ����ʵ���Щ |
| C��Ӧ�����浼�����ˮƽ |
| D��Ӧʹϸ�������浼��ƽ�� |









 ΪС���������������
ΪС���������������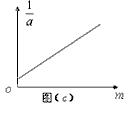
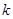 ���������ϵĽؾ�Ϊ
���������ϵĽؾ�Ϊ ����ţ�ٶ��ɳ�������С���ܵ�������Ϊ___________��С��������Ϊ___________��
����ţ�ٶ��ɳ�������С���ܵ�������Ϊ___________��С��������Ϊ___________��
 ��
�� ��
�� ��
�� ��
�� ������������ΪT�����E��ʱ�ٶ�Ϊ
������������ΪT�����E��ʱ�ٶ�Ϊ = �����ֱ��������������Ӧ���ٶ���ֵ����������ϵ�л���
= �����ֱ��������������Ӧ���ٶ���ֵ����������ϵ�л��� ��h�Ĺ�ϵͼ�ߣ���ͼ����ʾ�����������ٶ�
��h�Ĺ�ϵͼ�ߣ���ͼ����ʾ�����������ٶ�  =
=  ��
�� ����д��������Ҫԭ�� ��
����д��������Ҫԭ�� ��


