��Ŀ����
�����У�1������2������3������ѡ���⣬ÿ��7�֣��뿼����ѡ2����������14�֣������������������ǰ����Ƿ֣�����ʱ�����ڴ���ϰ���ѡ��Ŀ��Ӧ��������������У���1��ѡ��4-2��������任
��֪������
 ������ֵ��=-1����Ӧ��һ����������
������ֵ��=-1����Ӧ��һ���������� ��
�����������M��
����������C�ھ���M�������µõ��ķ���Ϊx2+2y2=1��������C�ķ��̣�
��2��ѡ��4-4������ϵ���������
��ֱ������ϵxOy�У�����C�IJ�������Ϊ
 Ϊ������������P���Ը�ֱ������ϵ��ԭ��O��Ϊ���㣬x���������Ϊ����ļ�����ϵ�µķ���Ϊp2-4pcos��+3=0��
Ϊ������������P���Ը�ֱ������ϵ��ԭ��O��Ϊ���㣬x���������Ϊ����ļ�����ϵ�µķ���Ϊp2-4pcos��+3=0������������C����ͨ���̺�����P��ֱ�����귽�̣�
����������C������P�Ľ���ΪA��B����|AB|��
��3��ѡ��4-5������ʽѡ��
��֪����f��x��=|x+1|+|x-2|������ʽt��f��x����x��R�Ϻ������
������ʵ��t��ȡֵ��Χ��
����t�����ֵΪT������ʵ��a��b��c����a2+b2+c2=T����a+2b+c�����ֵ��
���𰸡���������1������I�����ݾ��������ֵ�����������Ķ��彨����ʽ��ϵ����֮�������a��d��ֵ���Ӷ��������M��
��II�����A��x��y��Ϊ����C�ϵ���һ�㣬���ھ���M�������µõ��ĵ�ΪA'��x'��y'����Ȼ������ʽ��ϵ����A'��x'��y'�����뷽��Ϊx2+2y2=1������⼴�ɣ�
��2����������C�IJ�������Ϊ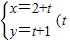 Ϊ����������ȥ����t������ͨ���̣��ٸ��ݼ������ֱ�����귽�̵Ļ�����ʽ�������C�ڼ�����ϵ�еķ��̣�
Ϊ����������ȥ����t������ͨ���̣��ٸ��ݼ������ֱ�����귽�̵Ļ�����ʽ�������C�ڼ�����ϵ�еķ��̣�
�����ɣ�I����ֱ��C�ļ����귽�̻�Ϊֱ�����귽�̣�������P�ļ����귽�̻�Ϊֱ�����귽�̣����Բ�ĵ�ֱ�ߵľ��룬�ٸ���Բ�İ뾶������ҳ���
��3����I��������֪����ʽt��f��x����x��R�Ϻ��������������f��x������Сֵ��ʹ��t��f��x��min���ɣ�
��II���ɣ���֪��T=3����a2+b2+c2=3���ɿ�������ʽ֪����a+2b+c��2�ܣ�a2+b2+c2����12+22+12�����������a+2b+c�����ֵ��
����⣺��1������С������7�֣�ѡ��4-2��������任
����������ã�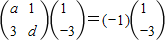 ����
����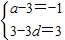 ������2�֣�
������2�֣�
���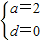 ������
������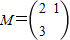 ������3�֣�
������3�֣�
����������C��һ��P��x��y���ھ���M�������µõ�����x2+2y2=1��һ��P'��x'��y'����
��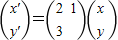 ����
����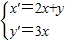 ������5�֣�
������5�֣�
����Ϊ��x'��2+2��y'��2=1�����ԣ�2x+y��2+2��3x��2=1��
����������C�ķ���Ϊ22x2+4xy+y2=1������7�֣�
��2������С������7�֣�ѡ��4-4������ϵ���������
��������C����ͨ����Ϊx-y-1=0������P��ֱ�����귽��Ϊx2+y2-4x+3=0������3�֣�
��������P�ɻ�Ϊ��x-2��2+y2=1����ʾԲ���ڣ�2��0�����뾶r=1��Բ��
��Բ�ĵ�ֱ��C�ľ���Ϊ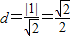 ������
������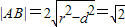 ������7�֣�
������7�֣�
��3������С������7�֣�ѡ��4-5������ʽѡ��
������ʽt��f��x����x��R�Ϻ��������t��f��x��min��
����Ϊf��x��=|x+1|+|x-2|��|��x+1��-��x-2��|=3�����Ժ���f��x������СֵΪ3��
����t��ȡֵ��ΧΪ��-�ޣ�3]������3�֣�
�����ɣ���֪��T=3����a2+b2+c2=3��
�ɿ�������ʽ֪����a+2b+c��2�ܣ�a2+b2+c2����12+22+12������a+2b+c��2��18��
����a+2b+c�����ֵΪ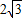 ������6�֣�
������6�֣�
���ҽ���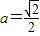 ��
��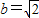 ��
��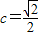 ʱ�Ⱥų���������7�֣�
ʱ�Ⱥų���������7�֣�
��������1����С����Ҫ���������任�������ھ���任�µ����ߵķ��̣������������������������ת��˼�룮
��2����С����Ҫ�������ߵIJ��������뼫���귽�̡�ֱ�ߵļ����귽�̵Ȼ���֪ʶ������������������Լ�������ת��˼�롢����������˼�룬���ڻ����⣮
��3����С����Ҫ�������������⡢��������ʽ�����ڻ������ͣ�
��II�����A��x��y��Ϊ����C�ϵ���һ�㣬���ھ���M�������µõ��ĵ�ΪA'��x'��y'����Ȼ������ʽ��ϵ����A'��x'��y'�����뷽��Ϊx2+2y2=1������⼴�ɣ�
��2����������C�IJ�������Ϊ
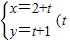 Ϊ����������ȥ����t������ͨ���̣��ٸ��ݼ������ֱ�����귽�̵Ļ�����ʽ�������C�ڼ�����ϵ�еķ��̣�
Ϊ����������ȥ����t������ͨ���̣��ٸ��ݼ������ֱ�����귽�̵Ļ�����ʽ�������C�ڼ�����ϵ�еķ��̣������ɣ�I����ֱ��C�ļ����귽�̻�Ϊֱ�����귽�̣�������P�ļ����귽�̻�Ϊֱ�����귽�̣����Բ�ĵ�ֱ�ߵľ��룬�ٸ���Բ�İ뾶������ҳ���
��3����I��������֪����ʽt��f��x����x��R�Ϻ��������������f��x������Сֵ��ʹ��t��f��x��min���ɣ�
��II���ɣ���֪��T=3����a2+b2+c2=3���ɿ�������ʽ֪����a+2b+c��2�ܣ�a2+b2+c2����12+22+12�����������a+2b+c�����ֵ��
����⣺��1������С������7�֣�ѡ��4-2��������任
����������ã�
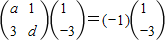 ����
����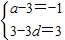 ������2�֣�
������2�֣����
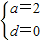 ������
������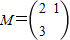 ������3�֣�
������3�֣�����������C��һ��P��x��y���ھ���M�������µõ�����x2+2y2=1��һ��P'��x'��y'����
��
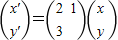 ����
����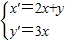 ������5�֣�
������5�֣�����Ϊ��x'��2+2��y'��2=1�����ԣ�2x+y��2+2��3x��2=1��
����������C�ķ���Ϊ22x2+4xy+y2=1������7�֣�
��2������С������7�֣�ѡ��4-4������ϵ���������
��������C����ͨ����Ϊx-y-1=0������P��ֱ�����귽��Ϊx2+y2-4x+3=0������3�֣�
��������P�ɻ�Ϊ��x-2��2+y2=1����ʾԲ���ڣ�2��0�����뾶r=1��Բ��
��Բ�ĵ�ֱ��C�ľ���Ϊ
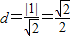 ������
������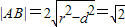 ������7�֣�
������7�֣���3������С������7�֣�ѡ��4-5������ʽѡ��
������ʽt��f��x����x��R�Ϻ��������t��f��x��min��
����Ϊf��x��=|x+1|+|x-2|��|��x+1��-��x-2��|=3�����Ժ���f��x������СֵΪ3��
����t��ȡֵ��ΧΪ��-�ޣ�3]������3�֣�
�����ɣ���֪��T=3����a2+b2+c2=3��
�ɿ�������ʽ֪����a+2b+c��2�ܣ�a2+b2+c2����12+22+12������a+2b+c��2��18��
����a+2b+c�����ֵΪ
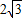 ������6�֣�
������6�֣����ҽ���
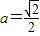 ��
��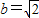 ��
��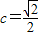 ʱ�Ⱥų���������7�֣�
ʱ�Ⱥų���������7�֣���������1����С����Ҫ���������任�������ھ���任�µ����ߵķ��̣������������������������ת��˼�룮
��2����С����Ҫ�������ߵIJ��������뼫���귽�̡�ֱ�ߵļ����귽�̵Ȼ���֪ʶ������������������Լ�������ת��˼�롢����������˼�룬���ڻ����⣮
��3����С����Ҫ�������������⡢��������ʽ�����ڻ������ͣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ