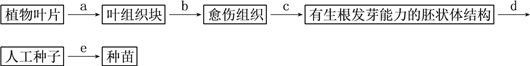题目内容
【题目】下面是果酒和果醋制作的实验流程和某同学设计的果酒和果醋的发酵装置。根据图示回答下列问题:
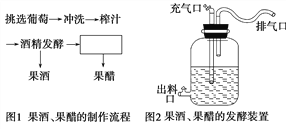
(1)完成图1中的实验流程。
(2)冲洗的主要目的是_______________________ ,冲洗应特别注意不能_____________,以防止菌种的流失。
(3)图2装置中的充气口在_____________时关闭,在______________时连接充气泵,并连续不断地向内_____________。葡萄汁装入发酵瓶时,要留有大约1/3的空间,这是因为_________________。
(4)排气口在果酒发酵时排出的气体是由 _____________产生的___________ ,在果醋发酵时排出的是含氧量少的空气和CO2 。
(5)写出两个与(4)题有关的反应方程式:
________________________________________________________
________________________________________________________。
(6)若在果汁中就含有醋酸菌,在果酒发酵旺盛时,醋酸菌能否将果汁中的糖发酵为醋酸?说明原因。 _____________________________________________________________。
(7)在酒精发酵时瓶内温度一般应控制为_____________ 。醋酸发酵时温度一般应控制为__________________________ 。
(8)若要提高果酒的产量,发酵过程中关键要控制好哪些条件?
______________________________________________________________
(9)腐乳制作中,起主要作用的生物是______________,它与乳酸菌在细胞结构上的主要区别是 ___________________ 。
(10)在腐乳制作中,加盐的目的是(至少写两个)________________、____________________ 。
【答案】 醋酸发酵 洗去浮尘 反复冲洗 果酒发酵 果醋发酵 泵入空气(氧) 既为酵母菌大量繁殖提供适量的氧气,又防止发酵旺盛时汁液溢出 酵母菌 CO2 C6H12O6![]() 2C2H5OH+2CO2 C2H5OH+O2
2C2H5OH+2CO2 C2H5OH+O2![]() CH3COOH+H2O 不能。因为果酒发酵时的缺氧能抑制醋酸菌生长,且醋酸菌发酵条件是氧气充足 18~25℃ 30~35℃ 适宜的温度、pH、通气量等(答出温度即可) 毛霉 毛霉有成形的细胞核 抑制微生物的生长,避免豆腐块腐败变质 加盐析出豆腐中的水分,使豆腐块变硬,在后期制作过程中不会过早酥烂 有调味作用
CH3COOH+H2O 不能。因为果酒发酵时的缺氧能抑制醋酸菌生长,且醋酸菌发酵条件是氧气充足 18~25℃ 30~35℃ 适宜的温度、pH、通气量等(答出温度即可) 毛霉 毛霉有成形的细胞核 抑制微生物的生长,避免豆腐块腐败变质 加盐析出豆腐中的水分,使豆腐块变硬,在后期制作过程中不会过早酥烂 有调味作用
【解析】试题分析:图1是果酒和果醋制作的实验流程,酒精发酵后还可以进行果醋发酵。图2是果酒和果醋的发酵装置图,其中排料口的作用是出料、检测;充气口的作用是在果醋制作时通入氧气的;排气孔是排气,其弯弯曲曲的好处是防止杂菌和浮尘的污染。
(1)果酒和果醋制作的实验流程是;挑选葡萄→冲洗→榨汁→酒精发酵→醋酸发酵→醋酸。
(2)冲洗的主要目的是洗去浮尘;制酒时用的菌种是葡萄表面的野生型酵母菌,因此冲洗时不能反复冲洗,以防止菌种的流失。
(3)制作果酒时需要无氧环境,而制作果醋时需要有氧环境,因此图2装置中的充气口在果酒发酵时关闭,在果醋发酵时连接充气泵,并适时向内泵入空气(氧)。葡萄汁装入发酵瓶时,要留有大约1/3的空间,既为酵母菌大量繁殖提供适量的氧气,又防止发酵旺盛时汁液溢出。
(4)排气口在果酒发酵时排出的气体是由酵母菌产生的二氧化碳;在果醋发酵时排出的是剩余的空气、二氧化碳。
(5)酒精发酵的反应式为:C6H12O6![]() 2C2H5OH+2CO2;醋酸发酵的反应式为:C2H5OH+O2
2C2H5OH+2CO2;醋酸发酵的反应式为:C2H5OH+O2![]() CH3COOH+H2O。
CH3COOH+H2O。
(6)若在果汁中就含有醋酸菌,在果酒发酵时的缺氧能抑制醋酸菌生长,且醋酸菌发酵条件是氧气充足,因此在果酒发酵旺盛时,醋酸菌不能将果汁中的糖发酵为醋酸。(7)在酒精发酵时瓶内温度一般应控制为18~25℃;醋酸发酵时温度一般应控制为30~35℃.(8)若要提高果酒的产量,发酵过程中关键要控制好适宜的温度、pH、通气量等。(9)腐乳制作中,起主要作用的生物是毛霉,毛霉(真核生物)与乳酸菌(原核生物)在细胞结构上的主要区别是毛霉有成形的细胞核。(10)在腐乳制作中,加盐的目的是抑制微生物的生长,避免豆腐块腐败变质;加盐析出豆腐中的水分,使豆腐块变硬,在后期制作过程中不会过早酥烂;有调味作用。