��Ŀ����
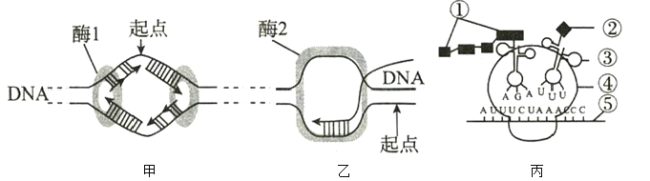
����Ŀ������ũ�˾�Ri������һ����Ȼ���ڵ�ֲ���Ŵ�ת��ϵͳ��Ri������3������������T-DNA����Ri������Ψһ���ϵ�ֲ��������DNAƬ�Σ���Vir���Ե������Ƿ���ũ�˾�ʵ�ָ�Ч��Ⱦ�����������Ori����������ʼ���빦�ܴ�л�������õ���������pCAMBIA1301���Էֱ��ڴ˾��Լ�����ũ�˾��н��п�¡������Ҫ������ͬԴ�����ڷ���ũ�˾��������ϼ������и��ơ����ֹ�����ͼ��ʾ��

�ش��������⣺
��1��������ũ�˾���Ⱦֲ��ʱ�����屾���������ϸ���ڣ�ֻ��___________________Ƭ�ν���ֲ��ϸ���У���ֲ��ϸ���н���������ϣ�����������_______________��������ֲ�����ڵ�øϵͳ����ת¼�ͷ��롣
��2��ѡ�����۵���ֲ����Ϊ����ϸ��ת��Ч��Զ���ڳ������ֲ�壬����ԭ����_______��
��3������������ø��ʶ��ͼ��LB/RB��ø��λ�㣬����ʹ______________________�Ͽ���
��4��Ӧ�ù����У�ֻ��Ҫ��Ŀ��Ƭ�β��뵽��������pCAMBIA1301�У��������õ�����ת��____________�У���ͨ��pCAMBIA1301������Я����___________________������DNA������ѡ��
��5������ũ�˾�Ri������Ϊһ����Ȼ���ڵ�ֲ���Ŵ�ת��ϵͳ����ʵ�����Ч��Ⱦ���������������Vir���Ե�����������Ҫ���ݴ��ƶ�Vir���Ե������ľ���������_______________��
���𰸡�Ri�����е�T-DNA ���壨ֲ�ϸ����Ⱦɫ��DNA�ϣ�����ϸ���Ļ������ϣ� ����ֲ��ϸ��������ʢ�ķ�������������ֲ��ϸ�����ڷ�����ʱ����������ԴDNA���� ������������֮��ģ���������� ����ũ�˾�������̬ϸ���� ����ù�أ����ԣ���ǣ����� ����ת�����ã���Ŀ�Ļ�����Ri������T-DNA��һͬ��������ֲ��ϸ����
��������
������ͼ�Ľ��Ϊ�龳������ѧ���Ի��̵Ļ���������������֪ʶ��ʶ�Ǻ������������Լ���ȡ��Ϣ�����������������
(1) ����ũ�˾�Ri�����ϵ�T-DNA��ת��������ϸ���������ϵ�����ϸ����Ⱦɫ���DNA�ϡ��ɼ���������ũ�˾���Ⱦֲ��ʱ�����屾���������ϸ���ڣ�ֻ��Ri�����е�T-DNAƬ�ν���ֲ��ϸ���У���ֲ��ϸ���н���������ϣ���������������ϸ����Ⱦɫ��DNA�ϣ�������ֲ�����ڵ�øϵͳ����ת¼�ͷ��롣
(2) ��������ֲ��ϸ��������ʢ�ķ�������������ֲ��ϸ�����ڷ�����ʱ����������ԴDNA���ϣ�����ѡ�����۵���ֲ����Ϊ����ϸ��ת��Ч��Զ���ڳ������ֲ�塣
(3) ����ø�ܹ�ʶ��˫��DNA���ӵ�ij���ض����������У�����ʹÿһ�������ض���λ������������֮���������������ѡ�
(4) ��ͼ��֪����Ӧ�ù����У���Ŀ��Ƭ�β��뵽��������pCAMBIA1301�У��������õ�����ת�뷢��ũ�˾�������̬ϸ�����С���������pCAMBIA1301���еij�ù�ؿ��Ի���Ϊ�����еı�ǻ���ǻ���������ǹ�����DNA�ļ�����ѡ��
(5) ��֪ Vir���Ե������Ƿ���ũ�˾�ʵ�ָ�Ч��Ⱦ����������ݴ˿���֪��Vir���Ե������ľ��������ǣ�����ת�����ã���Ŀ�Ļ�����Ri������T-DNA��һͬ��������ֲ��ϸ���С�

����Ŀ���������
�ɺ����οɵ�����в�ȣ�в��ָ��ֲ������˺��Ļ���������ʱֲ������Na+��Ϊ�о���ԭ������Ա������ֲ������Ҷ��Ϊ���ϣ���һ��ֲ�ﲻ�����õ��л���PEG��ģ����в�ȣ�������Һ�����Ӳ�ͬŨ�ȵ�NaCl�����о����NaCl����в���µ�����Ҷ��������Ӱ�졣��ͼΪ������ù���ʾ��ͼ��A��C��ʾ���ʣ��١��ڱ�ʾ���̡���Ϊʵ��10���Ľ����

��� | ���� | ֲ��������ӣ�g�� | ��������ʣ���molm-2s-1�� | Ҷ���غ�����mgg-1�� | ҶƬK+������mgg-1�� | ҶƬNa+������mgg-1�� |
A | Ӫ��Һ | 8.1 | 23.8 | 2.43 | 24.5 | 4.1 |
B | Ӫ��Һ+PEG | 3.0 | 0 | 2.02 | 24.5 | 4.0 |
C | Ӫ��Һ+PEG+10mmolL-1NaCl | 5.3 | 8.7 | 2.58 | 23.8 | 10.9 |
D | Ӫ��Һ+PEG+20mmolL-1NaCl | 7.1 | 15.3 | 2.61 | 23.4 | 16.8 |
E | Ӫ��Һ+PEG+40mmolL-1NaCl | 6.4 | 11.3 | 2.56 | 24.0 | 18.8 |
��1��ͼ����ĸA��ʾ______��B��ʾ______��
��2��������Ҷ��������еĹ�����______����ͼ�б�ţ�������Ծ��ѧ��ת��Ϊ�ȶ���ѧ�ܵĹ�����______����ͼ�б�ţ���
��3��������Ա��Ϊ��Ϊ��ʹʵ��������˵��������Ҫ������һ������Ķ���ʵ�飬���������Ϊ��������______��
A��Ӫ��Һ+20mmolL-1NaCl
B��PEG+20mmolL-1NaCl
C��Ӫ��Һ+80mmolL-1NaCl
D��PEG+80mmolL-1NaCl
��4�����ݱ��е�ʵ��������������˵����ȷ����______����ѡ����
A��B�������PEG����10�������Ҷ�����ٽ��й������
B��C��E���У�����Ҷ������ͨ������Na+��������ǿ��ˮ����
C������Ҷ���Կ���в����ҶƬK+������ϵ����
D��������NaCl���ܻ������Ҷ����������Ӱ��
��5�����ݱ��е�ʵ������˵������Һ������NaCl����в���µ�����Ҷ���ĸ���������������������Ӱ�죿������ԭ��______��