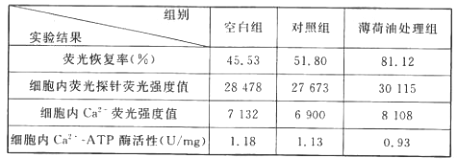
��Ŀ����
����Ŀ�����������������������е�һ�ࡣ���ݵ����������������ɹ�����������ͼ������ĸ��ʾ���Ŀ����£�ͨ�����ܺ���������ɵġ�������ش�

��1��ͼ���е�HVC�ȹ������������������仡�ṹ�е�____________���÷����ЧӦ����___________��
��2���о����������HVC��������Դ��ڴ���Ϊ̽�����ֲ����ԭ���о��߽���ʵ�飬�õ�ͼ����ʾ�Ľ������ͼ�����ش�
�ٴ�ʵ����____________����Ϊ���ϣ��Ʊ�װƬ��Ⱦɫ��Ȼ���������¹۲죬ͨ��������Ԫ��______________���ϸ���塱��ͻ�𡱣���ͳ��HVC����Ԫ��������
��ͼ�ҵĽ����ʾ��10��15����ʱ������ʹ����Ե�HVC����Ԫ���������첻������������������ʹ����Ե�HVC����Ԫ�����仯�����Ʒֱ�����������HVC����Ԫ�����������࣬��������١�
��3����һ���о����֣���������HVC������ϴ���������Ԫ��ռ�����ϸ��йأ���������Ԫ�����Ĵ��۲����������м����йأ����༤���������____________��
���𰸡� ������ ������ĩ�Һ�����֧������ܺ����� ��ͬ���䡢���Ժ����ԣ���ͬ����Ĵ�������� ϸ���� �Լ��أ����Լ��أ�
�������������������
������Ҫ�������ڵ����֪ʶ�����ڵĻ�����ʽ�Ƿ��䣬����Ľṹ�����Ƿ��仡�����仡ͨ���ɸ������������������ࡢ������ЧӦ����������ĩ�Һ�����֧��ļ��������ȣ���ɡ����۶�������ڵļ��ز�����Ҫ�������Լ��ء��ݴ˴��⡣
��1����ͼ��֪HCV�ڴ���Ƥ�㣬�Ƿ��仡�ṹ�е������ࣻ�÷����ЧӦ�����˶���ĩ�Ҽ�����֧������ܺ�������
��2������ͼ�Һ��������䣬������HCV��Ԫ��������֪����ʵ���Բ�ͬ���䡢���Ժ����ԣ���ͬ����Ĵ������������Ϊ���ϣ��Ʊ�װƬ��Ⱦɫ��Ȼ���������¹۲죬ͨ��������Ԫ��ϸ������ͳ��HVC����Ԫ��������
��3��������Ԫ�����Ĵ��۲����������м����йأ����༤����������Լ��أ����Լ��أ���

����Ŀ��ͼ1��ij��ֲ���CO2ͬ����ʽ�����յ�CO2����ƻ���ᴢ����Һ���У�Һ���е�ƻ���ᾭ���������ͷ�CO2���ڹ�����ã�ͼ2��ʾ��ͬ����A��B��C����ֲ���������ļ��Ĺ�������ձ仯���ߣ����ͼ�������ش�
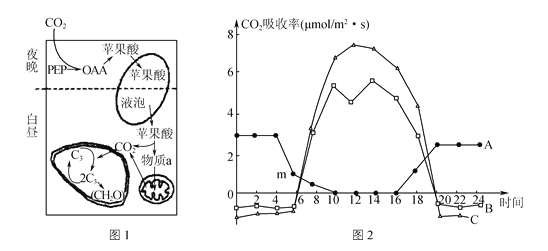
(1) ͼ1������ֲ���Ӧͼ2�е�________(����ĸ)��ֲ�ͼ1��ʾϸ����ҹ���ܲ���ATP�ij�����__________��ͼ1��ƻ�������Ⱥ�����Ŀɹ����������������a��________����ֲ��ҹ��������CO2��ȴ���ܺϳ�������л����ԭ����______________________��
(2) �����ж�ͼ1��������ֲ������������ƿ�ֲ����жϵ�������______________________��
(3) ������10��00ʱ��ͻȻ���ͻ�����CO2Ũ�Ⱥ��һС��ʱ���ڣ�ֲ��A��ֲ��Bϸ����C3�����仯�IJ�����____________��
(4) ͼ2��m��Ϊ����B��x�ύ�㣬Ӱ��m�������ƶ���������________��(��ѡ)
A. ֲ��ȱþ����B. �����¶�ʹ֮�����ˡ���C. ����ת��
D. CO2Ũ���ʵ��½�����E. CO2Ũ���ʵ����
(5) ʵ����̽������ǿ�ȶ�ֲ��C������л��Ӱ��ʱ�������ش�л�������±���
�ڰ�������CO2�ͷ��� | ����ǿ��Ϊ7.5 klxʱO2�ͷ��� |
1.2 ��mol/m2��s | 7.2 ��mol/m2��s |
������ǿ��Ϊ7.5 klxʱ��ֲ��C������ù̶���CO2����__________��