��Ŀ����
����Ŀ��ij��ѧ������ȤС����������������2,4��D��������ص�һЩʵ���о������������
(1)����ͬѧ��ȷ������������֦��������2,4��D��ҺŨ�ȣ����������ֲ�ͬŨ�ȵ�2,4��D��Һ������ˮ�ֱ�������״����ͬ������֦�������������������֦������״�����첻�����������������飬��ʹ���ǴﵽԤ��Ŀ�ġ�
________________________________________________________________________��
(2)����ͬѧΪ̽��2,4��D��ҺŨ�ȶ��¼�֦��������Ӱ�죬���Ȱ���ͼһ��ʾ����������һϵ��Ũ���ݶȵ�2,4��D��Һ��Ȼ��ѡ����������顢��ţ�����֦�������������������õ�ʵ������ͼ����ʾ��
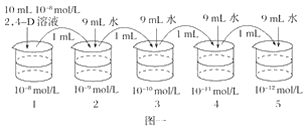
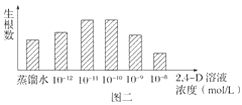
����ͼ������֪����5���ձ��е�2,4��D��Һ�IJ�����Ӧ________________���ò���������________________ԭ��
����ͼ����ȷ���ٽ��¼�֦������������2,4��D��ҺŨ�ȵķ�Χ��__________________��ʵ����________(�ܣ�����)���������ԡ�
���𰸡���1������������Ũ��2��4��D��Һ�Ļ����ϣ��ֱ��ڵ��ڵ�Ũ�ȡ����ڸ�Ũ���Լ���Ũ��֮������һϵ��Ũ���ݶȵ�2��4��D��Һ����ʵ�飬�ֱ�۲�����״�����ҵ�����������֦��������2��4��D��ҺŨ�ȡ� ��2��������1ml ��һ������������ ��10-11mol/L��10-10mol/L ��
��������
����
(1)��������û�дﵽԤ��Ŀ�ģ�����ԭ�л����϶����ü���Ũ���ݶȣ��ֱ��ڵ��ڵ�Ũ�ȡ����ڸ�Ũ���Լ���Ũ��֮������һϵ��Ũ���ݶȵ�2.4��D��Һ����ʵ�飬�ֱ�۲�����״�����ҵ�����������֦��������2.4��D��ҺŨ�ȣ�
(2)����ͼ�й۲죬Ϊ��ѭʵ��ĵ���ԭ��5���ձ��е�2��4-D��Һ�Ļ�Ӧ����1ml���ò���������ʵ��ĵ���ԭ��ͬʱҲ������ʵ��ĵ�һ����ԭ��
����ͼ���۲��ȷ���ٽ��¼�֦������������2,4��D��ҺŨ����10-11mol/L��10-10mol/L֮�䣻��ͼ���������������ԣ��������������ں�������ˮ�Ķ������Ϊ�ٽ������ڵ�Ϊ���ơ�