题目内容
【题目】细胞呼吸分有氧呼吸和无氧呼吸,两者进行的场所、过程等均存在差异。下图表示细胞部分结构和功能,据图回答:
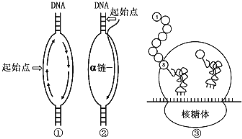
(1)图中A物质是 ,B物质在该过程的产生部位是 。
 (2)在有氧呼吸过程中,产生C在第____________阶段。产生能量最多是在第____________阶段。
(2)在有氧呼吸过程中,产生C在第____________阶段。产生能量最多是在第____________阶段。
(3)如果有氧呼吸和无氧呼吸产生等量的CO2,所消耗的葡萄糖之比是__________________。
(4)图中能体现的无氧呼吸方程式应该是:________________________。
(5)图中未能体现的无氧呼吸方程式应该是:________________________。
(6)图中产生B物质的呼吸作用方程式为:_________________________。
【答案】
(1)丙酮酸 线粒体内膜
(2)二 三
(3)1:3
(4)C6H12O6![]() 2C2H5OH+2CO2+能量
2C2H5OH+2CO2+能量
(5)C6H12O6![]() 2C3H6O3+能量
2C3H6O3+能量
(6) C6H12O6+6H2O+6O2![]() 2CO2+12H2O+能量
2CO2+12H2O+能量
【解析】
(1)图中A物质是是第一阶段产物所示是丙酮酸,B物质是水在该过程的产生部位是线粒体内膜
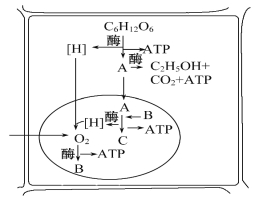 (2)有氧呼吸的第一阶段的葡萄糖酵解产生丙酮酸和[H],同时释放少量能量,发生在细胞质基质中,第二阶段是丙酮酸与水反应产生二氧化碳和[H],同时释放少量能量,发生在线粒体基质中,第三阶段是[H]与氧气生成水,释放大量能量的过程,发生在线粒体内膜上。
(2)有氧呼吸的第一阶段的葡萄糖酵解产生丙酮酸和[H],同时释放少量能量,发生在细胞质基质中,第二阶段是丙酮酸与水反应产生二氧化碳和[H],同时释放少量能量,发生在线粒体基质中,第三阶段是[H]与氧气生成水,释放大量能量的过程,发生在线粒体内膜上。
(3)如果有氧呼吸和无氧呼吸产生等量的CO2,所消耗的葡萄糖之比是1:3。
(4)图中能体现的无氧呼吸方程式应该是:C6H12O6![]() 2C2H5OH+2CO2+能量
2C2H5OH+2CO2+能量
(5)图中未能体现的无氧呼吸方程式应该是:C6H12O6![]() 2C3H6O3+能量。
2C3H6O3+能量。
(6)图中产生B物质的呼吸作用方程式为:C6H12O6+6H2O+6O2![]() 2CO2+12H2O+能量。
2CO2+12H2O+能量。

 学业测评一课一测系列答案
学业测评一课一测系列答案【题目】现有两种淀粉酶A与B,某生物兴趣小组为探究不同温度条件下这两种淀粉酶的活性,设计如下探究实验:
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
Ⅰ、设置水浴缸温度(oC) | 20 | 30 | 40 | 50 | 20 | 30 | 40 | 50 | |
Ⅱ、取8支试管各加入淀粉溶液10(mL),分别保温5分钟 | |||||||||
Ⅲ、另取8支试管各加入等量淀 粉酶溶液,分别保温5分钟 | 酶A | 酶A | 酶A | 酶A | 酶B | 酶B | 酶B | 酶B | |
Ⅳ、将同组两个试管中的淀粉溶液与淀粉酶溶液混合摇匀,保温5分钟。 | |||||||||
实验结果:对各组淀粉剩余量进行检测,结果如下图所示。
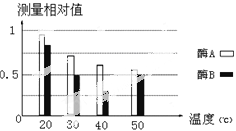
(1)该实验的自变量是 和 。
(2)根据实验结果分析,酶B在 ℃条件时活性较高。
(3)本实验能不能用斐林试剂检测生成物麦芽糖的含量来表示? ,(1分)理由是 。
(4)若要进一步探究酶B的最适温度,实验设计的主要思路应是在 ℃之间设立较小的温度梯度的分组实验,按上述步骤进行实验,分析结果得出结论。
【题目】成熟的植物细胞具有中央大液泡,可与外界溶液构成渗透系统进行渗透吸水或渗透失水。下图甲表示渗透装置吸水示意图,图乙表示甲图中液面上升的高度与时间的关系,图丙表示成熟植物细胞在某外界溶液中的一种状态(此时细胞有活性)。请回答下列问题:

(1)由甲图漏斗液面上升可知,实验初始时c两侧浓度大小是a b(大于或等于或小于)。由图乙可知漏斗中溶液吸水速率在_________,最终液面不再上升,当液面不再上升时,c两侧浓度大小是a b(大于或等于或小于)。
(2)丙图中相当于甲图中c的组合结构是__________(填序号),结构②当中充满的液体是___________。此时细胞液的浓度与外界溶液的浓度大小关系是_________。
A.细胞液 < 外界溶液 | B.细胞液 > 外界溶液 |
C.细胞液 = 外界溶液 | D.都有可能 |
(3)把一个已经发生质壁分离的细胞浸入低浓度的蔗糖溶液中,发现细胞液泡体积也在增大。当液泡体积不再增大时,细胞液浓度是否一定和外界溶液浓度相等?___________。原因是_____________________。