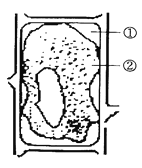
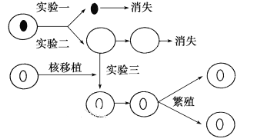
��Ŀ����
����Ŀ��ij�ߵȶ������ڵ�ϸ�����ѹ��̿�����ͼ����ʾ������ͼ�ױ�ʾϸ�����Ѹ���Ⱦɫ�������DNA���ı�����ϵ����ʵ�߱�ʾ����ϸ������mRNA�����ı仯�������߱�ʾ����ͼ�ұ�ʾ�ö��2n=4��������AaBb��ijһ�����ڵ�ϸ������ͼ�����ͼ�ش�

��1����ϸ�����ѷ�ʽ����ͼ���Ա�ʾ__________________���ѡ�ͼ����d��e��ϸ������mRNA���Լ��٣�����ܵ�ԭ����__________________________��
��2��ͼ��ϸ������ͼ���У�������λ������롢�ǵ�λ����������ϵ�ϸ����_________������ͼ��BC�ε�ϸ����_______________��
��3��ͼ����BC�Σ�ϸ���д��ڵ�Ⱦɫ����������Ϊ________________�����ס�����ʾΪͬһ��������ϸ������ͼ�����Ӧͼ����BC�ε�ϸ�����ܴ��ڵ�XȾɫ������Ϊ_____________��
���𰸡���˿���ѻ�������� ������ϸ������DNA�����ڸ߶���������Ⱦɫ���У����ܽ�����ת¼��mRNA(���߷����ڲ���ת¼) III III���� 1������2�� 1����0��
��������
����ּ�ڿ���ϸ�����ѷ�ʽ���жϣ�ϸ�����Ѹ���Ⱦɫ�������DNA���ı�����ϵ��ͼ�η�������ͼ��֪��AB�Σ���d��Ⱦɫ�������DNA���ı�ֵ��1��Ϊ1/2����ʾS�䣨��DNA�ϳ�ʱ�ڣ�����a��ʾG1�ڣ�DNA�ϳ�ǰ�ڣ���c��ʾG2�ڣ�DNA�ϳɺ��ڣ���d��eΪ�����ڣ�CD���γɵ�ԭ������˿����ѡ���ͼ�ҷ�����Iϸ������ͬԴȾɫ������������������Ⱦɫ��û��Ⱦɫ���壬Ϊ��˿���Ѻ��ڣ�IIϸ��Ϊԭʼ��ֳϸ����IIIϸ����ͬԴȾɫ�����ڷ��룬���ڼ�����һ�η��Ѻ��ڣ���ϸ���ʾ��֣�˵���øߵȶ���Ϊ���ԣ���ϸ������ͬԴȾɫ�壬��ʾ�����ڶ��η���ĩ�ڵľ�ϸ���������Զ������ڼȽ�����˿�����ֽ��м������ѵ�����Ϊغ�衣
��1����˿���Ѻͼ������Ѷ�����ͼ��G1�ڡ�S�ڡ�G2�ڡ������ڣ����Դ�ϸ�����ѷ�ʽ����ͼ���Ա�ʾ��˿���ѻ�������ѷ��ѡ�d��e�δ��ڷ����ڣ���ʱϸ�����е�DNA�����ڸ߶���������Ⱦɫ���У����ܽ�����ת¼�γ�mRNA������ϸ������mRNA���Լ��١�
��2��ͼ��ϸ������ͼ���У�IIIϸ����ͬԴȾɫ�����ڷ��룬��ͬԴȾɫ��������ϣ���������λ������롢�ǵ�λ����������ϵ�ϸ����ͼ��BC�μ�d��e�δ��ڷ���������ʾ��˿����ǰ�ڡ����ڻ������һ�η��ѹ��̡������ڶ��η���ǰ�ں����ڣ���ͼ���д��ڷ����ڵ�ϸ���Т�ϸ����
��3��ͼ����BC�α�ʾ��˿����ǰ�ڡ����ڣ�Ⱦɫ������Ϊ2�����������һ�η��ѣ�Ⱦɫ������Ϊ2�������̡������ڶ��η���ǰ�ں����ڣ�Ⱦɫ������Ϊ1����������ϸ���д��ڵ�Ⱦɫ����������Ϊ1������2�������ס�����ʾΪͬһ���弴���ԣ���Ⱦɫ�����ΪXY������ϸ������ͼ�����Ӧͼ����BC�α�ʾ��˿����ǰ�ڡ����ڻ������һ�η��ѹ���ʱ��ϸ�����ڵ�XȾɫ������Ϊ1����BC�α�ʾ�����ڶ��η���ǰ�ں�����ʱ������ͬԴȾɫ����룬ϸ���ڴ��ڵ�XȾɫ������Ϊ1����0������Ӧͼ����BC�ε�ϸ�����ܴ��ڵ�XȾɫ������Ϊ1����0����

����Ŀ����(Cd)��һ�ֶ��Ժܴ���ؽ���Ԫ�أ����ֲ�����������˺����������Ϊ����̽����Դ��(Ca)�ܷ�Cd�Ķ�����
��1��ʵ�鲽�裺
�� ������(25 ��)�����£�������ˮ��������۾������ճ���ҶƬ��ѡȡ80������״��һ�£�ֲ��߶Ȼ�����ͬ���������ƽ���ֳ�________�飬���α�š�
ÿ���Ӵ����ƴ�����Ũ������������������������ͬ�����ˡ�
��� | �Ӵ���(��mol/L) | ||||
0 | 10 | 100 | 300 | ||
�ƴ���(mmol/L) | 0 | A1 | B1 | C1 | D1 |
0.1 | A2 | B2 | C2 | D2 | |
1 | A3 | B3 | C3 | D3 | |
10 | A4 | B4 | C4 | D4 | |
�� ���ֱܺ�______________________________��
��2������ʵ��������ͼ��ͼ��ʾ��
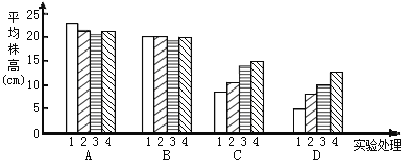
ʵ����������ۣ�
�� A1��B1��C1��D1����ʵ����˵������_______�����ٽ�����������������е�������
�� A��B��ʵ����˵�����ڵ���Ũ�������£���ԴCa����е����������Ե�Ӱ�죻��C��D��ʵ������˵�������С�����Ũ�������£���Դ���ܲ���____������ǿ���������������Ӷ����������ɵ��������ã��Ҹ�Ũ��Խ_____������������������������Ч��Խ����
�� ��һ���о����֣�Ca2����Cd2������ϸ��������������ͨ��������Һ��Ca2����Cd2��ͬʱ����ʱ��Ca2����������____________________���Ӷ������ӵĶ�����
�� �������й������ӱ���е�ֲ�����ջ��ۣ���ͨ��ʳ�������ݽ������壬ʹ��������еĸƴ�����ʧ���ٴ��ϳ�����________________���������ƣ��Դٽ����峦���ԸƵ����ա��ٴ��ϲ���Ĵ���������____________�ķ�ʽ����ϸ����