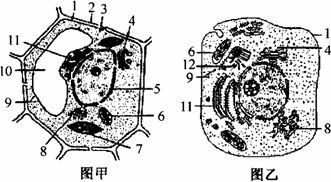
��Ŀ����
ͼ��ϸ���ǽ�ͼ��ϸ��������һ��Ũ��������Һ�еĽ����ͼ���ǽ�����һ��ֲ���ϸ�����ν�������ˮ��0.3 mol/L���Ǻ�0.5 mol/L������Һ�У��۲�ԭ���ʲ�������ʱ��仯�Ľ�������ͼ�ش�
 |
��1����ͼϸ���У�ȫ�ԵĽṹ��___����ͼϸ���а��Ե�ԭ���ʲ��� ���ɣ����ţ���
��2����ͼϸ�����ʱڷ������ʱ���ദ������
��3����ͼABC�У���ʾϸ��������ˮ�е��� ����ʾ��0.3 mol�ML������Һ�е��� ��
��4��bc�ε�ϸ��ҺŨ�ȱ仯Ϊ ��������С�����䣩
��5���Լ�Ҫ����B��C���߲����ԭ��B����Ϊ��0.5mol�ML������Һ�е�ϸ����ʼʱ����ʧˮ�������ʱڷ��룬������ �ܽ���ϸ����ʹ���ʱڷ�����ϸ������ˮ���Զ���ԭ��C���� ���ܽ���ϸ������˷�����ܷ����Զ���ԭ��
��1��2 11 ��12 ��13
��2��������Һ
��3��A C
��4����С
��5������ ����

 ��У����ϵ�д�
��У����ϵ�д���25�֣�
��6�֣���ͼΪ����ij��֯��ʾ��ͼ�����ͼ�ش��������⣺

��1��ͼ��a��b��c ������ϸ����Һ���������з����зֱ���д�����Ʋ��ü�ͷ��ʾ��a��b��c ����֮��Ĺ�ϵ��
 |
��2�������ڵ�ϸ��ͨ�� ��������绷����ӵؽ������ʽ�������ˣ�ֻ��ά��ϸ�������Һ�廷������̬��������ܽ����������������Ŀǰ�ձ���Ϊ�� ���������ǻ���ά����̬����Ҫ���ڻ��ơ�
��3����ʱ�����ߺŵ�ĥ��ˮ�ݡ�������ˮ�ݣ������ĵ���ɫҺ����Ҫ�� ��
��11�֣���ͼ��ʾ���ַ�����ؽṹ��ͼ����ͼ����ijһ�ṹ�������ṹģʽͼ����ͼ�ش�

(1)��ͼ��f��ʾ�Ľṹ�������� ������ͼ�Ǽ�ͼ����������������ĸ���������ṹ�Ŵ�ģʽͼ����ͼ�е�B����һ����Ԫ������ ����������
��2��[��]��������_________��Ϊ���˷ܵĴ����ṩ������ϸ����[ ]_________�����ַ���ʱ���˷ܴ�A����B���ź����������� �����˷ܲ�����B����A��ԭ������������������ ��
��3��ͻ����Ĥ�ϵġ����塱����Ӧ���ʽ�ϣ�����Bϸ��������������������ʹͻ����Ĥ��Ĥ��λ�����仯���ṹ���������ͷŵ�ͻ����϶�Ĺ���������ϸ��Ĥ�Ľṹ�ص���� ��
��4��[��]�ṹ��������____________��������άδ�ܴ̼�ʱ���ô�ϸ��ĤĤ_______�����ڻ��⣩Ϊ����λ��
��8�֣���ѧ�ҽ�ʹơ�Ʋ����ḻ��ĭ��LTP1����ֲ��ơ�ƽ�ĸ���У�ʹ�����LTP1���ף������ĭ�ḻ��ơ�ƣ�����IJ�����������ͼ��ʾ����ע������������ø���ʶ�����к��е��ǡ�G��GATCC��������������ø���ʶ�����к��е��ǡ���GATC����

��1����ͼ����ʾ�Ļ��̲������̵�A��C�ֱ���_____________��______________��
��2������ͼ�п��Կ������õ�������B��__________��
��3�����뻭��LTP1��������ø���и�����γɵ����ĩ�ˣ�______________________��
����LTP1�����B���γɵ����ĩ���ܷ��ϣ�__________�������ܵĻ�����������Ҫ��һ�ֹ���ø
_________ _____��
��4������μ��LTP1������ơ�ƽ�ĸ���еı��__________________ _______________��
��5�����ù������Ŵ���Ϣ�Ĵ��ݹ����� ��
������4�֣���д����ͼ: �����γɵĹ���

��ش������й�������ѧʵ������⡣
��1�������ˮ��ϸ���ıȽϹ۲�ʵ�飬��ͼ�м������� ���õ�Һ�Լ�����ϸ������Ⱦɫ����Ⱦɫ��������жϸ��������� ���

��2��ͼ������ĸA�����Ľṹ��Ⱦɫ���__________ɫ��ϸ���д�״�ṹ�ϵ�B�����ʣ���Ⱦɫ���__________������____________________��
��3���ڵͱ������߱����¿���������и���ϸ��ͼ�������۲�ֲ��ϸ������˿���ѡ�ʵ��ʱ����Ҫ�۲�ͼ _______����ϸ����ͼBϸ�����������Ϊ _____����




A B




C D
��4��ijͬѧ���±���ʾ�Ƴ���ʱװƬ�����й�ʵ�顣
| ��� | ���� | ʵ������ | �۲����� |
| A | ��ɫ�����ƬҶ��� Ƥ | ��ˮ������Һ��0��3g��ml������Һ | �ʱڷ��� |
| B | ��ɫ�����ƬҶ���Ƥ | 0��3g/ml������Һ | �ʱڷ��� |
��ش��������⣺
������ʵ�������������μ�������Һ��Ŀ���� ____________��
����A��ʵ���У������¿������ʱڷ�������ԭ���� ____���ƻ���ʧȥ��ѡ�����ԡ�
��5�����ã�4����B��ʵ�鷽���ⶨij��ֲ��Ҷ��Ƥϸ��Һ��Ũ�ȡ��ֱ�Ƭ��ͬ��Ҷ��Ƥ���ڲ�ͬŨ�ȵ�������Һ�У�10���Ӻֱ�ÿһҶƬ��Ƥ�Ƴ���ʱװƬ���������¹۲졣��Լ20��ϸ������Ұ��Χ�ڣ��Գ����ʱڷ����ϸ�����м��������ý�����±���
| ����Ũ�ȣ�mol��dm3�� | �ʱڷ���ϸ������Ŀ | ��Ұ��Χ�������ӵ�ϸ������ |
| 0��00 0��05 0��10 0��15 0��20 0��25 0��30 0��35 | 0 0 0 1 3 16 19 2l | 22 20 18 20 20 20 19 21 |
�������ϱ��Ҳ����꣬��ȷ�ػ��һ����ͼ���Ա�ʾ�����ʱڷ���ϸ���İٷ�����������ҺŨ�ȵĹ�ϵ��

�����������������ͼ�У����ҳ�������ҺŨ��Ϊ0��22mol��dm3ʱ�������ʱڷ���ϸ���İٷ���Ϊ ��