��Ŀ����
����Ŀ��ţ�̷���ʱ�䳤�˻���ᣬ������Ϊţ���к��е�����������������·ֽ��������ᡣ����������Ǵ���ţ���еõ����ɴ˶������ġ�����Ľṹ��ʽΪCH3CH(OH)COOH������������⣺
(1)д�����������������Ʒ�Ӧ�Ļ�ѧ����ʽ��__________��
(2)���ᷢ�����б仯��
![]()
![]()
![]()
![]()

���õ��Լ���a________��b________(д��ѧʽ)��д������������̼������Һ��Ӧ�Ļ�ѧ����ʽ��___________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���͡�
�������Ҵ���Ӧ��__________��________��
(4)������Ũ���������£����������Ӧ������Ԫ��״�ṹ�����ʣ�д����������Ľṹ��ʽ_______��
���𰸡�CH3CH(OH)COOH+2Na��CH3CH(ONa)COONa+H2�� NaHCO3 Na CH3CH(OH)COOH+ NaHCO3��CH3CH(OH)COONa+CO2��+H2O CH3CH(OH)COOH+CH3CH2OH![]() CH3CH(OH)COOCH2CH3+H2O ������Ӧ����ȡ����Ӧ��
CH3CH(OH)COOCH2CH3+H2O ������Ӧ����ȡ����Ӧ�� 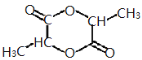
��������
������������ǻ����Ȼ���������д������ʣ�������������ʡ�
(1)���������������Ʒ�Ӧ�Ļ�ѧ����ʽΪCH3CH(OH)COOH+2Na��CH3CH(ONa)COONa+H2����
(2)![]()
![]()
![]() ���÷�Ӧ��ֻ���Ȼ����뷴Ӧ�����ǻ������룬�����õ��Լ�a��NaHCO3��
���÷�Ӧ��ֻ���Ȼ����뷴Ӧ�����ǻ������룬�����õ��Լ�a��NaHCO3��
![]()
![]()
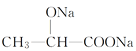 ���÷�Ӧ�����ֹ����ž����뷴Ӧ�������õ��Լ�b��Na������������̼��������Һ��Ӧ�Ļ�ѧ����ʽΪCH3CH(OH)COOH+ NaHCO3��CH3CH(OH)COONa+CO2��+H2O��
���÷�Ӧ�����ֹ����ž����뷴Ӧ�������õ��Լ�b��Na������������̼��������Һ��Ӧ�Ļ�ѧ����ʽΪCH3CH(OH)COOH+ NaHCO3��CH3CH(OH)COONa+CO2��+H2O��
(3)�������Ҵ����Է���������Ӧ��������������ˮ���÷�Ӧ����ȡ����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��CH3CH(OH)COOH+CH3CH2OH![]() CH3CH(OH)COOCH2CH3+H2O��
CH3CH(OH)COOCH2CH3+H2O��
(4)������Ũ���������£����������Ӧ������Ԫ��״�ṹ�Ľ������˽����Ľṹ��ʽΪ ��
��