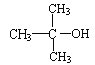
��Ŀ����
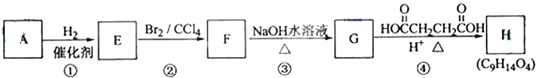
����Ŀ��A����H��ת����ϵ������ʾ��
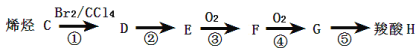
��ش��������⣺
��1������A��֧����ֻ��һ�������ţ�����Է���������65��75֮�䣬1molA��ȫȼ������7mol��������A�Ľṹ��ʽ�� �������� ��
��2�����ض����������£�A������ʵ�����H2��Ӧ����E����Eת��ΪF�Ļ�ѧ����ʽ�� ��
��3��G������Ʒ�Ӧ�ܷų����塣��Gת��ΪH�Ļ�ѧ����ʽ�� ��
��4�����ķ�Ӧ������ �����ķ�Ӧ������ ��
��5������B��A��ͬ���칹�壬�����е�����̼ԭ�ӹ�ƽ�棬����⻯����Ϊ�����飬д��B���п��ܵĽṹ��ʽ ��
���𰸡���1��(CH3)2CHC��CH��3__��__1__��Ȳ
��2��CH2=CHCH(CH3)2+Br2��CH2BrCHBrCH(CH3)2
��3��![]()
��4���ӳɷ�Ӧ(��ԭ��Ӧ)��ȡ����Ӧ
��5��CH3CH=CHCH=CH2��CH3CH2C��CCH3
��������
�������������A��֧����ֻ��һ�������ţ�����Է���������65-75֮�䣬1molA��ȫȼ������7mol������A�ܷ����ӳɷ�Ӧ��˵������̼̼�����ͼ�����A�ķ���ʽΪCxHy��65��12x+7y��75��x+![]() =7������x=5��y=8��A�ķ���ʽΪC5H8������A��֧����ֻ��һ�������ţ���A�Ľṹ��ʽΪCH��CCH(CH3)2�����ض����������£�A������ʵ�����H2��Ӧ����E����E�ṹ��ʽΪCH2=CHCH(CH3)2��B���巢���ӳɷ�Ӧ����F��F�ṹ��ʽΪCH2BrCHBrCH(CH3)2��F����ˮ�ⷴӦ����G��G�ṹ��ʽΪHOCH2CH(OH)CH(CH3)2��G�Ͷ����ᷴӦ����H��H�ṹ��ʽΪ
=7������x=5��y=8��A�ķ���ʽΪC5H8������A��֧����ֻ��һ�������ţ���A�Ľṹ��ʽΪCH��CCH(CH3)2�����ض����������£�A������ʵ�����H2��Ӧ����E����E�ṹ��ʽΪCH2=CHCH(CH3)2��B���巢���ӳɷ�Ӧ����F��F�ṹ��ʽΪCH2BrCHBrCH(CH3)2��F����ˮ�ⷴӦ����G��G�ṹ��ʽΪHOCH2CH(OH)CH(CH3)2��G�Ͷ����ᷴӦ����H��H�ṹ��ʽΪ![]() ��
��
��1��A�Ľṹ��ʽ��CH��CCH(CH3)2��������3-��-1-��Ȳ���ʴ�Ϊ��CH��CCH(CH3)2��3-��-1-��Ȳ��
��2��A�Ľṹ��ʽΪCH��CCH(CH3)2��E�ṹ��ʽΪCH2=CHCH(CH3)2��F�ṹ��ʽΪCH2BrCHBrCH(CH3)2����Eת��ΪF�Ļ�ѧ����ʽ��CH2=CHCH(CH3)2+Br2��CH2BrCHBrCH(CH3)2���ʴ�Ϊ��CH2=CHCH(CH3)2+Br2��CH2BrCHBrCH(CH3)2��
��3��G�ṹ��ʽΪHOCH2CH(OH)CH(CH3)2��G�Ͷ����ᷴӦ����H��H�ṹ��ʽΪ![]() ����Gת��ΪH�Ļ�ѧ����ʽ����
����Gת��ΪH�Ļ�ѧ����ʽ����![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��4�����ķ�Ӧ�����Ǽӳɷ�Ӧ�����ķ�Ӧ������ȡ����Ӧ��ˮ�ⷴӦ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��ˮ�ⷴӦ
��5������B��A��ͬ���칹�壬�����е�����̼ԭ�ӹ�ƽ�棬����⻯����Ϊ�����飬��B�к���һ��̼̼����������̼̼˫����������ϩ����Ȳ�Ľṹ֪��B���п��ܵĽṹ��ʽ��CH3CH=CHCH=CH2��CH3CH2C��CCH3���ʴ�Ϊ��CH3CH=CHCH=CH2��CH3CH2C��CCH3��