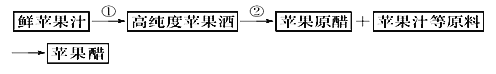
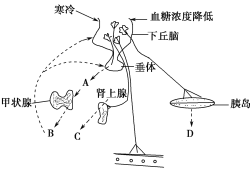
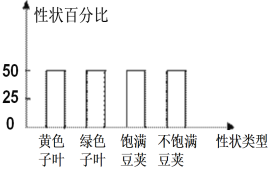
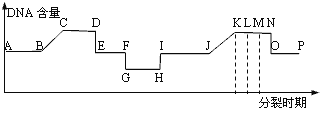
��Ŀ����
����Ŀ����ɫ���ij�Ⱦɫ������һϵ�о�����ë��ɫ�ĸ���λ������G��gch��gh��g���û���ϵ���ھ�����ë��ɫʱ��������������͵Ĺ�ϵ���±�����ش��������⣺

��1�������ë��ɫ�Ļ������� �֡������Ӻ����� �֡�
��2����һֻ����ɫ����һֻdz��ɫ��콻������µ�С�������ë��ɫΪ����ɫ������ɫ=1:1,����ֻ�ױ����Ļ����ͷֱ�Ϊ �� ��
��3��������ɫ������콻������������ɫ�Ͱ�ɫ��죬�����Ӵ��е�����ɫ��dz��ɫ�Ӻ��彻�䣬����ı����ͼ����� ��
��4������һֻdz��ɫ�����Ͷ�ֻ������ɫ�Ĵ���죬ʵ��ԱӦ��ѡ�ø�dz��ɫ������� ���䣬����� ,���dz��ɫ�����Ļ�����Ϊ ������� �����dz��ɫ�����Ļ�����Ϊ ��
���𰸡���1��10 6
��2��Ggch ghgh �� ghg
��3�����ϣ�dz�ϣ���=4��1:1
��4����ֻ��ɫ����� ��Ϊdz��ɫ��죬ghgh �����˰�ɫ�����dz��ɫ����ɫ=1: 1����ghg
��������
�����������1�������ë����ɫ�У�����ɫ�Ļ�������GG��Ggch��Ggh��Gg��4�֣�����ɫ�Ļ�������gchgch��gchgh��gchg��3�֣�dz��ɫ�Ļ�������ghgh��ghg��2�֣���ɫ�Ļ�����Ϊgg��1�֣��ʻ�������10�֣����Ӻ���ΪGgch��Ggh��Gg��gchgh��gchg��ghg����6�֡�
��2������һֻ����ɫ�����G_����һֻdz��ɫ�����gh_����������µ�С�����ë��ɫΪ����ɫ��G_��������ɫ��gch_��=1��1����������ɫ����gchֻ����������ɫ��죬��������ɫ���Ļ�����ΪGgch����dz��ɫ���Ļ����Ϳ���Ϊghgh��ghg��
��3��������ɫ������콻����gch_��gch_���������������ɫ��gch_���Ͱ�ɫ��gg����죬˵���״�����ɫ���Ļ����;�Ϊgchg��������������Ļ����ͼ�����Ϊgchgch������ɫ����gchg������ɫ����gg����ɫ��=1��2��1���������ɫ����У����ֻ����ͼ�����Ϊ1/3gchgch��2/3gchg��������Ⱥ���е�dz��ɫ�Ӻ�����ghg�����䣬����1/3gchgch��������Ϊ����ɫ��2/3gchg������Ϊ2/3��1/2����ɫ��1/4dz��ɫ��1/4��ɫ������˺���ı����ͼ����������ϣ�dz�ϣ�������1/3+2/3��1/2����2/3��1/4��2/3��1/4=4��1��1��
��4��ѡ�ö�ֻ��ɫ�Ĵ���죬���dz��ɫ����콻�䣬�������Ϊdz��ɫ��죬���dz��ɫ�����Ļ�����Ϊghgh������������˰�ɫ��죬���dz��ɫ�����Ļ�����Ϊghg��

����Ŀ��������һ����Ҫ��ũҵ���ϣ���������ϸ���ķֽ⣬�Ͳ��ܸ��õر�ֲ�����á������������е�����������������࣬ͬѧ����ͼ̽������������������Ƿ��зֽ����ã����������ʵ�飬���ɹ�ɸѡ���ܸ�Ч�������ص�ϸ����Ŀ�ľ������������ɷ����±���ʾ��ʵ�鲽������ͼ��ʾ��������ش�
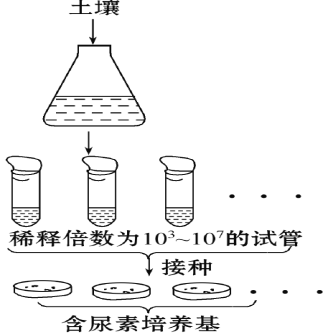
KH2PO4 | Na2HPO4 | MgSO4��7H2O | ������ | ���� | ��֬ | ||||||
1��4 | g | 2��1 | g | 0��2 | g | 10 | g | 1 | g | 15 | g |
�����������ܽ��������ˮ���ݵ�1 000 mL��
��1�����������ʷ֣�������������______ ��
��2���������м������ص�Ŀ���ǣ� �������������� ��������
��3����Ŀ�ľ�����������ĵ�Դ��̼Դ�ֱ������������е� �� ��ʵ����Ҫ��ԭ���� ��
��4��ͼ�н�ϸ��ת��������������ʱ���ɲ���____________���֣���õ���������ɸѡ��
��5����ʵ���У����в��ϻ��þ���Ҫ������� ����Ҫ�������� ��������ţ�
������ϸ���õ���������������
���������Թܡ���ƿ������
��ʵ������ߵ�˫��
��6���ڽ��з���ֽ����ص�ϸ��ʵ��ʱ��Aͬѧ����������ɸѡ����Լ150�����䣬������ͬѧֻѡ�����Լ50�����䡣Aͬѧ��ʵ����������ԭ�������____________������ţ���
��������ͬ ����������Ⱦ
������ʧ��