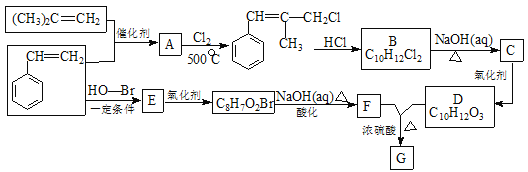
��Ŀ����
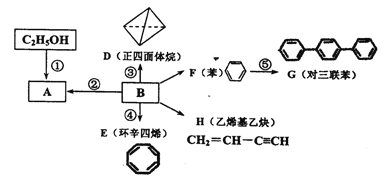
����Ŀ���л���G������ʽΪC16H22O4���dz��õ�Ƥ�����������Ⱥϳɲ����е�������������һ�ֺϳ�·������ͼ��ʾ��
��֪���� A��һ�ֳ��������ĺ��������A������ͼ������ʺɱ�Ϊ46����˴Ź�������ͼ��������壬�ҷ����֮��Ϊ1��2��3 ��
�� E�DZ���ͬϵ���Է���������100��110֮�䣬��E�����ϵ�һ�ȴ���ֻ��2�֣�![]()
�� 1molF�������ı���NaHCO3��Һ��Ӧ�ɲ�������44.8L����״���£���
����R1��R2��ʾ��ԭ�ӻ���������
�����������Ϣ�ش��������⣺
��1��A������Ϊ_______��д��A ��һ��ͬ���칹��Ľṹ��ʽ_________��
��2��Aת��ΪB�Ļ�ѧ����ʽΪ__________________________��
��3��C�����������ŵ�����Ϊ_________
��4��E�Ľṹ��ʽΪ_____________��
��5��D��F��Ӧ����G�Ļ�ѧ��Ӧ����ʽΪ______________________��
��6��F�ж���ͬ���칹�壬д��ͬʱ��������������F��ͬ���칹����_____�֡�
�� �ܺ�NaHCO3��Һ��Ӧ���� �ܷ���������Ӧ���� ��FeC13��Һ����ɫ��
��7����������������Ϣ�ͼ�֪��Ϣ��������Ҵ��ͱ���Ϊԭ�ϣ����Լ���ѡ���Ʊ�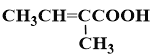 �ĺϳ�·��_______________���ϳ�·������ͼʾ�����£�
�ĺϳ�·��_______________���ϳ�·������ͼʾ�����£�
���𰸡� �Ҵ� CH3OCH3 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ̼̼˫����ȩ��
2CH3CHO+2H2O ̼̼˫����ȩ�� ![]()
![]() 10
10 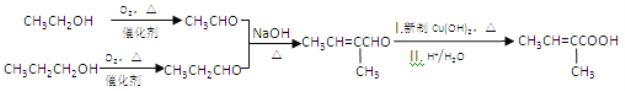
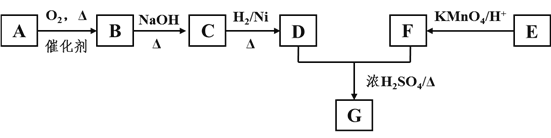
��������A��һ�ֳ��������ĺ��������A������ͼ������ʺɱ�Ϊ46����˴Ź�������ͼ��������壬�ҷ����֮��Ϊ1:2:3��AΪCH3CH2OH���Ҵ�������������ȩ��������Ϣ����ȩ���������Ƽ���ʱ����C��CΪCH3CH=CHCHO��C�������ӳ�����D��DΪCH3CH2CH2CH2OH��E�DZ���ͬϵ���Է���������10��110֮�䣬��������F��1molF�������ı���NaHCO3��Һ��Ӧ�ɲ���2mol������̼����F�к���2���Ȼ������EΪ���ױ���E�����ϵ�һ�ȴ���ֻ��2�֣�EΪ![]() ����FΪ
����FΪ![]() ��
��
(1)��������������AΪCH3CH2OH���������Ҵ����Ҵ�����ѻ�Ϊͬ���칹�壬�ʴ�Ϊ���Ҵ��� CH3OCH3��
(2)�Ҵ�������������ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(3)CΪCH3CH=CHCHO��C��������������̼̼˫����ȩ�����ʴ�Ϊ��̼̼˫����ȩ����
(4)EΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)DΪCH3CH2CH2CH2OH��FΪ![]() ������������Ӧ����G�Ļ�ѧ��Ӧ����ʽΪ
������������Ӧ����G�Ļ�ѧ��Ӧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)FΪ![]() ���� �ܺ�NaHCO3��Һ��Ӧ��˵�������Ȼ����� �ܷ���������Ӧ��˵������ȩ����Ϊ���������� ��FeC13��Һ����ɫ��˵�����з��ǻ�������������F��ͬ���칹����
���� �ܺ�NaHCO3��Һ��Ӧ��˵�������Ȼ����� �ܷ���������Ӧ��˵������ȩ����Ϊ���������� ��FeC13��Һ����ɫ��˵�����з��ǻ�������������F��ͬ���칹����![]() ��
��![]() (����λ�ÿɱ�)�ȣ��ʴ�Ϊ��
(����λ�ÿɱ�)�ȣ��ʴ�Ϊ��![]() ��
��![]() ��
��
(7)���Ҵ��ͱ���Ϊԭ���Ʊ�![]() �����Խ��Ҵ�����������ȩ���������������ɱ�ȩ��������Ϣ��������CH3CH=C(CH3)CHO,����������������������Ϊ
�����Խ��Ҵ�����������ȩ���������������ɱ�ȩ��������Ϣ��������CH3CH=C(CH3)CHO,����������������������Ϊ![]() ������ͼΪ
������ͼΪ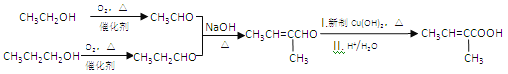 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

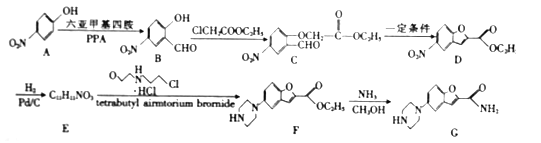
����Ŀ��ij��ȤС��ͬѧ��ʵ�����ü���l��������ŨH2SO4���廯�ƻ����ķ������Ʊ�1���嶡�飬���������ͼ��ʾ��ʵ��װ�������еļг�����û�л�������

��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���� ������װ���ж��õ��������ܣ�Aװ������ˮ�� ������ĸ���������룬Bװ������ˮ�� ������ĸ���������롣
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
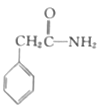
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ�����CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� ��
��4��Ϊ�˽�һ���ᴿ1���嶡�飬��С��ͬѧ�������л�����й��������±���
���� | �۵㣯�� | �е㣯�� |
1������ | ��89.5 | 117.3 |
1���嶡�� | ��112.4 | 101.6 |
���� | ��95.3 | 142.4 |
1����ϩ | ��185.3 | ��6.5 |
����Bװ����ɴ��ᴿʵ��ʱ��ʵ����ҪѸ�������¶��� �ռ�������֡�
����Ŀ��ʵ�����Ʊ������������Ļ�ѧ����ʽ
![]()
�Ʊ������л����ܵĸ���Ӧ��
![]()
��Ҫʵ�鲽�����£�
���ڸ����Բ����ƿ�м�11.5mL��������7.2mL�������3~4��ŨH2SO4��ҡ�ȺӼ�����ʯ������ͼ1װ�ð�װ�÷�ˮ���������ܡ�Ȼ��С����ȡ�

�ڽ���ƿ�з�Ӧ��Ļ������ȴ���ˮ���е�����ϲ���ת���Һ©����
��������10mLˮ��10mL 10%̼������Һϴ�������ԣ�pH=7������ˮϴһ�Σ���������ˮ����þ���
�ܽ�����������������ת��50mL������ƿ�У��Ӽ�����ʯ���г�ѹ�����ռ���Ʒ����Ҫ�Լ������������������
������ | ������ | ������ | ���������� | ������ |
�ܶ�(g��cm-3) | 0.810 | 1.049 | 0.882 | 0.7689 |
�е㣨�棩 | 118.0 | 118.1 | 126.1 | 142 |
����������Ϣ�ش��������⡣
��1��ͼ��װ������ˮӦ�� ����a��b�����ܿ�ͨ�룬��Ӧ���������������ͨ����ˮ���¶������ֳ����������� ����Ŀ���� ��

��2���ڲ���٢��ж��õ���ʯ�����У����й��ڷ�ʯ��˵����ȷ���� ��
A��ʵ���ҷ�ʯҲ�������Ƭ�ȴ���
B��������ӷ�ʯ�����ٽ���ʯ�����������ڵ�Һ����
C�������½�������ʱ���ù��ķ�ʯ�ɼ���ʹ��
D����ʯΪ��������ʣ���ʹҺ��ƽ�ȵط���
��3���ڲ���۷�Һʱ��Ӧѡ��ͼ��װ���� �η�Һ©������ԭ���� ��
��4�����ᴿ�ֲ�Ʒ�Ĺ����У���̼������Һϴ����Ҫ��ȥ������ ������������������Һ�Ƿ���� ������ԡ������ԡ�������ԭ�� ��
��5������ܵij�ѹ�������ռ� �����֣��е����140����л������������һ�㲻�����������ܶ��ÿ��������� ������ԭ���� ��