��Ŀ����
��ش��������⣺���о��������˵�ABOѪ�Ͳ�����λ��9��Ⱦɫ���ϵ�IA��IB��i�������������λ�ڵ�19��Ⱦɫ���ϵ�H��h�����йء��������ڣ�ǰ��������H������������γ�H���ʣ���hh���˲��ܰ�ǰ������ת���H���ʡ�H������IA����������£��γ�����ԭA��H������IB������������γ�����ԭB����ii���˲���ת��H���ʡ���ԭ����ͼ1��ʾ��

��1����������ԭ������������ԭB����Ӧ����_________�����_________����
��2��ij��ϵ��ϵ��ͼ��ͼ2��ʾ����-2�Ļ�����ΪhhIBi����ô��-2�Ļ�������__________��
��3��һ�Ի�����ΪHhIAi�ķ���Ѫ��ΪO��Ѫ�ĺ��ӵļ�����__________��
�Ҳϵ��Ա����ΪZW�ͣ���������ΪZZ������ΪZW�����������׳��Ƥ���������ɻ���A���ƣ����Ͳϡ��׳��Ƥ��������ֽ�����Կ����ڲ����٣����ɻ���a���ƣ����Ƕ�ֻλ��ZȾɫ���ϡ���ɣ�������з��֣��۲ϲ�˿�ࡢ�����ã�Ϊ�����׳�ʱ���ܼ�ʱ������ۣ��Ա���̭�Ʋϣ������۲ϣ������һ���ӽ�ʵ�飨��ͼ���ʾ����������Ҫ˵�������������Ϻ͡��Ͳϡ�����Ʒ�ִ����׳������������
������
������1��H��IB
������2��HhIAIB
������3��7��16
����������ͼ��ʾ���Ӵ��з������Ͼ�Ϊ���ԣ�ZAZa�������ͲϾ�Ϊ���ԣ�ZaW�������׳�ʱ���ɽ��������֡�
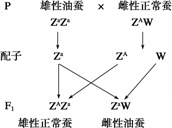

��10�֣��������Ⱦɫ�������ZZ���������Ⱦɫ�������ZW��ij������ë����ɫ�ɳ�Ⱦɫ�����A��a������Ⱦɫ�����ZB��Zb����ͬ�������������������͵Ķ�Ӧ��ϵ���±�����ش��������⡣
| ������� | A�����ڣ�����B�������aa Z��Z����aa Z��W�� | A���ڣ�B������ ��A ZbZb��A ZbW�� | A��Bͬʱ���� ��A ZBZ����A ZBW |
| ��ë��ɫ | ��ɫ | ��ɫ | ��ɫ |
��2�������ʹ��ϵĻ��������Ӻϵĺڴ����䣬�Ӵ��д������ɫ�� ���������ɫ�� ��
��3����ֻ�����䣬�Ӵ���ëֻ�к�ɫ�Ͱ�ɫ�����Ļ�����Ϊ ��ĸ���Ļ�����Ϊ ��
(4)һֻ��������һֻ�Ҵ����䣬�Ӵ���ë�к�ɫ����ɫ�Ͱ�ɫ����ĸ���Ļ�����Ϊ �������Ļ�����Ϊ ����ɫ����ɫ�Ͱ�ɫ�Ӵ������۷����Ϊ ��
��9�֣���ĸ�����������ճ������з�����Խ��Խ������á���ش��������⣺
��1����ĸ�����������ʹ���������������� ��
| A��ţ��൰���������� | B����ѿ֭��֬������ |
| C�������˽�ĸ�������� | D��MS������ |
�ٹ�֭���ͺ��Ƿ�����ƾ��������� �����飬�����������£���������ƾ���Ӧ�� ɫ��
�ڽ�ĸ��ϸ���̶����ɴ���������Ч�ʡ��̶���ĸ��ϸ��ʱ������Ҫ���ɽ�ĸ���� �л��
��3���⽫��ɸѡ��õ�������ƽ�ĸ��������,��ĸ����Ⱥ�����ı仯���±���ʾ�������þ���һ��ѡ�ÿ�����ֵ�εľ���,�ɱ������ݷ���,�� ��Ľ�ĸ���������ڹ̶�����
| ʱ�䣯�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| ��ĸ��������106��mL�� | 0��8 | 1��8 | 3��0 | 5��6 | 4��8 | 2��0 | 0��8 | 0��4 |
��5��������ˮ����ʳ��Ӫ���������ܣ��Ǽ�Ӫ����������ʳ�Ƶȹ���Ϊһ���������Ʒ��������ijͬѧ��ƵĹ��ƺ���������ʵ�����̡�

��ϴӦ�ر�ע�ⲻ�ܷ�����ϴ��ԭ���� ��
��ͼ��֪�������������ڹ������������Ͻ��У�������ط�Ӧʽ��ʾ��һ���̣� ��
��6��С�������Ժ��д�������ѣ���Ҫ�ɷ�Ϊľ���ء���ά�غͰ���ά�أ����ǽ�ĸ����ֱ�����ã�ԭ������ ��


 ��������Ƥ��ɫ��Ҳ���뷢��Һ��ʹ���ѾƳ������ɫ���� �ķ���Һ�У���ĸ������������ֳ������������������ﶼ������Ӧ��һ�������ܵ����ơ�
��������Ƥ��ɫ��Ҳ���뷢��Һ��ʹ���ѾƳ������ɫ���� �ķ���Һ�У���ĸ������������ֳ������������������ﶼ������Ӧ��һ�������ܵ����ơ� ת��Ϊ ������������������¶�Ϊ ��
ת��Ϊ ������������������¶�Ϊ ��