��Ŀ����
�������ѡ��1���\��ʵ������15�֣�
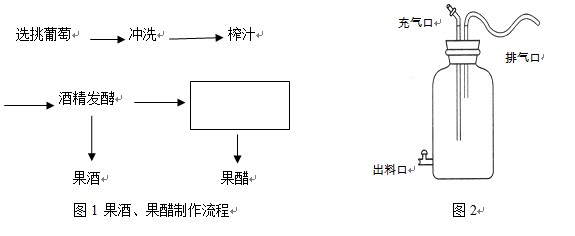
��������������������ơ�������ʷԴԶ��������ش��������⣺
��1�����Ƶ������벻����ĸ������ĸ����һ�� ���������ͣ���������������£���ĸ�����������������䷴ӦʽΪ �������������£���ĸ�����������������䷴ӦʽΪ ��
��2���¶��ǽ�ĸ�������ͷ��͵���Ҫ������20���������ʺϽ�ĸ����ֳ���ƾ�����ʱһ�㽫�¶ȿ����� �������ѾƵ���Ȼ�������У�����Ҫ���õ��Ǹ���������Ƥ�ϵ�Ұ���ͽ�ĸ�����ڷ������У����� ����� ��������Ƥ��ɫ��Ҳ���뷢��Һ��ʹ���ѾƳ������ɫ���� �ķ���Һ�У���ĸ������������ֳ������������������ﶼ������Ӧ��һ�������ܵ����ơ�
��������Ƥ��ɫ��Ҳ���뷢��Һ��ʹ���ѾƳ������ɫ���� �ķ���Һ�У���ĸ������������ֳ������������������ﶼ������Ӧ��һ�������ܵ����ơ�
��3���������һ�ֺ���ϸ����ֻ�е���������ʱ�����ܽ�����ʢ����������ڱ���ľƵı���۲쵽�ľ�Ĥ���Ǵ������Һ�������ֳ���γɵġ�ʵ�������������������ĺ��� ������������Դ������ʱ�������������֭�е��Ƿֽ�� ����ȱ����Դʱ����������Ҵ���Ϊ ���ٽ�һ�� ת��Ϊ ������������������¶�Ϊ ��
ת��Ϊ ������������������¶�Ϊ ��
��4����֭���ͺ��Ƿ��оƾ������������û�ѧ�Լ� �����顣�����������£����Լ���ƾ���Ӧ���� ɫ��
��1������������1�֣� C6H12O6 + 6O2��6CO2 + 6H2O��2�֣� C6H12O6��2C2H5OH + CO2��2�֣�
��2��18��25�棨1�֣� �ƾ�������1�֣� ȱ���������ԣ�1�֣�
��3���ر����У�1�֣� ���ᣨ1�֣� ��ȩ��1�֣� 30��35�棨1�֣� ���ᣨ1�֣�
��4���ظ���أ�1�֣� ����ɫ��1�֣�
����

 �������⣬������������������______________��_________�Լ�___________��Ҫ��
�������⣬������������������______________��_________�Լ�___________��Ҫ��