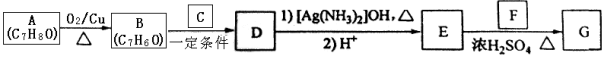��Ŀ����
����Ŀ��x��y��z��w �������ʾ����������ֻ����е�������϶��ɡ�
��H��C6H5������OH����CHO����COOH����CH3
��1��ȼ�� 0.1molx������ 2.24 ��������̼(��״��������x ������̼���Ʒ�Ӧ������������Ʒ�Ӧ��x �Ľṹ��ʽ��______________________________��
��2��y ������̼�����Ʒ�Ӧ��������̼���Ʒ�Ӧ���ڿ����лᱻ����������һԪȡ���������ֽṹ���� y �Ľṹ��ʽ��______________________________��
��3��z �������ԣ�z �����Ⱦͷֽ�����壬z �Ľṹ��ʽ��____________________��
��4��w �������³���̬��w �����к�������Ԫ�أ�w �Ľṹ��ʽ��__________��
��5����һ�������£�x��y��z��w ����֮���ܷ�����ѧ��Ӧ�Ļ�ѧ����ʽ�ǣ�____________��
���𰸡�
��1��CH3OH
��2��C6H5OH
��3��HO-COOH
��4��HCHO
��5��![]()
��������
�����������1�� 2.24 ��������̼�����ʵ���Ϊ0.1mol��x ������̼���Ʒ�Ӧ������������Ʒ�Ӧ��˵��x�к����ǻ��������Ȼ�����x�к���1��̼ԭ�ӣ���x�ĽṹΪCH3OH���ʴ�Ϊ��CH3OH��
��2��y ������̼�����Ʒ�Ӧ��������̼���Ʒ�Ӧ��˵��y�Ƿ��࣬�� y ΪC6H5OH���ʴ�Ϊ��C6H5OH��
��3��z �������ԣ�z �����Ⱦͷֽ�����壬z Ϊ̼�ᣬ�ʴ�Ϊ��HO-COOH��
��4��w �������³���̬��w �����к�������Ԫ�أ�w Ϊ��ȩ���ʴ�Ϊ��HCHO��
��5����һ�������£�x��y��z��w ����֮���ܷ�����ѧ��Ӧ���DZ��Ӻͼ�ȩ���Է�Ӧ���ɷ�ȩ��֬����ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��
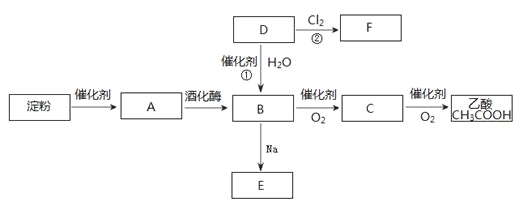
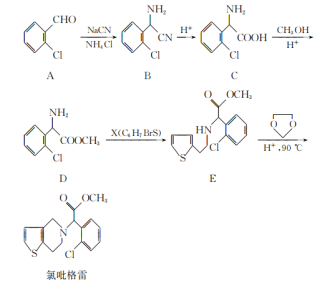
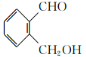
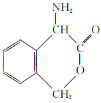
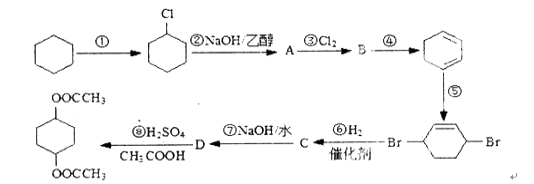
��1��д��C�Ľṹ��ʽ ��
��2����Ӧ�������� ����֪F�Ľṹ����˫������F�Ľṹ��ʽΪ ��
��3��д��B�����ᷢ�������Ļ�ѧ����ʽ ��
��4�����ࡢ��֬�������ʾ�Ϊ������Ҫ��Ӫ�����ʣ�����˵����ȷ���ǣ� ��
A��������A��������Һ��Ϻ�ˮԡ��������������˵��A�д���ȩ�� |
B����֬�Ǹ�֬��������γɵ�����߷��ӻ��������������ж����ڰ������Ȼ����ֹ����� |
C�����̶���С���ƿ�Ͷ��B�У��ƿ鸡��Һ���ϣ����д������ݲ��� |
D�����������Ʊ�ʵ�����ñ���̼������Һ�ռ���������������²� |