��Ŀ����
����Ŀ����ش��������⣺
��1����ͬһֲ��ϸ�����ν�������ˮ��0.3 mol/L��������Һ��0.4 mol/L��KN03��Һ�У����ϸ���������ʱ��ı仯������ͼ��ʾ��
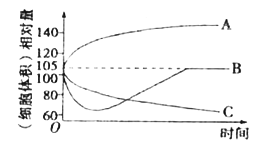
������A��B��C�ֱ����ϸ����������Һ��__________��__________��__________��
�ڴﵽƽ���__________��Һ��ϸ����ϸ��ҺŨ�����
��2����ͼ��ij�������С���ڹ۲�ֲ��ϸ���ʱڷ����ԭʵ��ʱ���۲쵽�ļ�����Ƭ��������ѧ��֪ʶ��������ͼ���ش����⣺

�ٷ����ʱڷ������__________����������ʹ�Ѿ��ʱڷ����ֲ��ϸ�������ʱڷ��븴ԭ��
������������Ƭ��ʾ����ͬһ��֯��ϸ������ʱ��Ƭ1��ϸ��Һ��Ũ��_______������ڡ���С�ڡ�����Ƭ2��ϸ��Һ��Ũ�ȡ�
���𰸡� ����ˮ KNO3��Һ ������Һ ���� ��������ˮ С��
��������������ʵ���ͼ�ο���ֲ��ϸ������ˮ��ʧˮ��Ҫ������������ֲ��ϸ����һ����ϵͳ���ܷ����ʱڷ�����ʱڷ��븴ԭ������ȷϸ����ˮ�ᵼ��ϸ��ҺŨ�Ƚ��ͣ�ϸ����ˮ�������½�����ϸ��ʧˮ�ᵼ��ϸ��ҺŨ�����ߣ�ϸ����ˮ��������ǿ��ͬʱ������KNO3��Һ�������ϸ��Ϊʲô���ȷ����ʱڷ�����������Զ���ԭ��ԭ��
��1���ٳ����ֲ��ϸ����һ����ϵͳ�����з��������õ���������ϸ��ҺŨ�ȴ��������Һ������ˮ��Ũ��ʱ��ֲ��ϸ����ͨ����������ˮ������ϸ������������ͼ������A����ϸ����������Һ������ˮ����ϸ��ҺŨ��С�������ҺŨ��ʱ��ֲ��ϸ����ͨ��������ʧˮ������ϸ�������С��0.3mol/L��������Һ��0.4mol/L��KN03��Һ������ϸ��ҺŨ�ȣ�ϸ�����������������Һ��ʱ����ʧˮ������ϸ�������С����ֲ��ϸ����ͨ����������ķ�ʽ����K+��N03-��������ϸ��Һ��Ũ�ȣ���˴���0.4mol/LKN03��Һ�е�ֲ��ϸ��������ˮ���Զ���ԭ����������Һ�е�ֲ��ϸ�������Զ���ԭ�����ͼ������B����ϸ����������Һ��0.4mol/L��KN03��Һ������C�ֱ����ϸ����������Һ��0.3mol/L��������Һ��
��ֲ��ϸ����ˮ��ϸ��ҺŨ�Ƚ��ͣ�ֲ��ϸ��ʧˮ��ϸ��ҺŨ������������������֪���ﵽƽ�������ˮ��ֲ��ϸ���Ѿ���ˮ��һ����ˮ�֣������ϸ��ҺŨ�ȱȳ�ʼ״̬�ͣ�������Һ�е�ֲ��ϸ��ʧȥ��һ����ˮ�֣������ϸ��ҺŨ�ȱȳ�ʼ״̬�ߣ�KN03��Һ�е�ֲ��ϸ����ʧˮ����������K+��N03-���Զ���ԭ�������ϸ��ҺŨ�����ʼ״̬��ͬ�����Ϸ�����֪���ﵽƽ���������Һ��ϸ����ϸ��ҺŨ�����
��2���ٷ����ʱڷ����ϸ��ҺŨ�ȱ��ʱ�õ�Ũ�ȵ������Һ������ˮ����������ʹ�Ѿ��ʱڷ����ֲ��ϸ�������ʱڷ��븴ԭ��
������������Ƭ��ʾ����ͬһ��֯��ϸ�����ɼ���ʱ��Ƭ1��ϸ����������״̬���ʱڷ��븴ԭ״̬��������Ƭ2��ϸ������ʧˮ״̬���ʱڷ���״̬���������Ƭ1��ϸ��Һ��Ũ��С����Ƭ2��ϸ��Һ��Ũ�ȡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�