��Ŀ����
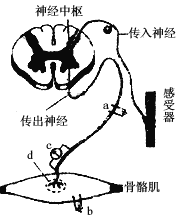
����Ŀ����״��ϸ�����Խ�������͵�ϳ�Ϊ��״���ף����ҽ���״�����ڵ�ϸ���⣬���������ͼ��ʾ��������������ȷ����( )
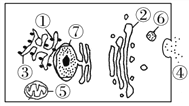
A. ���״�����ڹ����йصľ�Ĥ��ϸ�����Ǻ����塢���������߶����塢������
B. b�������ȷ����ڢ���
C. �ú�3H��ǵİ�����ע�䵽��ϸ���У������3H�IJ�λ����Ϊ�٢ۢڢޢ�
D. ����18O�İ������ڼ�״��ϸ���ڵĴ�л�����в�����H![]() O����ôˮ�е�18O����������ڰ�����ġ�COOH
O����ôˮ�е�18O����������ڰ�����ġ�COOH
���𰸡�A
���������������Dz���Ĥ�ṹ��ϸ������A���ӱ�ǵİ��������ϸ���ϳɵ����ʵ����ڵ�ϸ���⣬���ξ����IJ�λ�Ǣٺ����塢�����������ڸ߶����塢�����ݡ���ϸ��Ĥ��B��ȷ��C���������-COOH�к���O����л����ת����ˮ�е�O��D��ȷ��

����Ŀ��ij�����Ի�ֲ�������ɫ�����Ե�λ����H��h��R��r�����ƣ���������뻨����ɫ�Ķ�Ӧ��ϵ���±���
������� | H_RR | H_Rr | ���������� |
������ɫ | ��ɫ | �ۺ�ɫ | ��ɫ |
�ش��������⣺
��1��������ΪHhRr��ֲ�꣬���Ի�����Ⱦɫ���ϵ�λ�ù�ϵ���������֣�����ռ�ͼ�����������������ͣ����߱�ʾȾɫ�壬Բ���ʾ����
���ͱ�� | �� | �� | �� |
������Ⱦɫ���ϵ�λ�� |
| ____________ | ____________ |
��2��ȡ������ΪHhRrֲ��Ļ��ۣ�����������������õ����羭��ˮ���ش������������������ڣ�������ֲ��ȫ����ɫ�����������Ⱦɫ���ϵ�λ�ù�ϵ�����____________��ʾ��
��3�������Խ�ʵ����̽�������Ի�����Ⱦɫ���ϵ�λ�ù�ϵ����Ӧѡ�û�����Ϊ________��ֲ������Խ�������Ӵ�ֲ��ı����ͼ�������________��˵�������Ի���λ��________����ѭ����������϶��ɡ�
��4���������Ի�������Ŵ����Ի�����HhRr��ֲ��Ϊ�ױ��Խ���F1�����п���ɫ����ֲ�����Խ�����F2�п���ɫ����ֲ����ռ�ı���Ϊ________��
����Ŀ��������Ǹߵ�ֲ��㷺���ڵ�����������ֲ����Ӧ�����仯��һ�����֡��о������������������ˮ���ĸ��ᷢ��������������������ԡ���Ϊ�о�IAA��ˮ������������˶���Ӱ�켰�й����û������о���Ա��������ص�ʵ�顣
��1����֪Ca2+��Ϊ�źŷ��ӣ���ֲ��Ķ����ź�ת������������������������Ҫ�����á�Ϊ̽��Ca2+�Ƿ��Ӱ�쵾����IAA�ķֲ����о���Ա�ü���H2O��CaCl2��Һ��LaCl2��Һ��Ca2+ͨ����ϼ����Լ��������Һ����������Һ�ֱ�����ˮ������ճ����ĸ����ڵ��������24h�����鵾�������ָ�����ԣ�ÿ�����IAA�ķֲ��������ͼ��
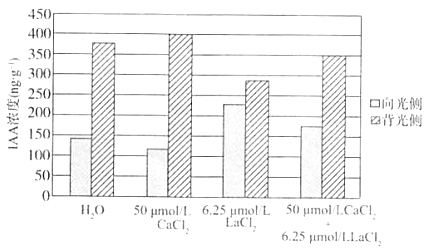
�ɽ����֪���ڵ�������¶�������IAA�ķֲ������_________����Ca2+��Ϊ�źŷ���_______�����������ȣ�������ʵ��Ľ������Ca2+��__________���Ӷ���һ��֤��Ca2+�ڸ���������˶��ж�IAA�ֲ���Ӱ�졣
��2��Ŀǰ��֪cpt1��������CPT1������ˮ����ѿ��������˶�������IAA�����������Ҫ���塣Ϊ̽��cpt1�����Ƿ���ˮ������������˶��йأ��о���Ա��ˮ������ճ����ĸ��ֱ���в�ͬ������24h��������������ȣ�����������������±�����ͬʱ�о���Ա���ⶨ�˸��鵾����cpt1������������������ͼ����
�����Լ���Ũ�� | �����Ƕ� | |
�ڰ� | ������� | |
1mgL-1CaCl2 | 0 | 42.9 |
1mgL-1LaCl2 | 0 | 26.0 |
0.001mgL-1IAA | 0 | 38.1 |
H2O | 0 | 36.1 |
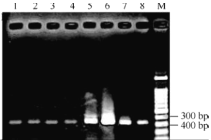
��ͼ�е�1��4��ʾ�ڰ�������cpt1����ı�������7��ʾH2O������ı�������5��6��8Ӧ�ֱ���________�����Ľ�����ɴ˿�֪����Դʩ�ӵ������Լ��Ե�����cpt1�����������Ӱ�������ǶԵ��������ȵ�Ӱ����һ�µġ��ڴ�ʵ���������ϣ�������о���Ա�ⶨ�ĵ��������cpt1����ȱʧͻ����ˮ����������ͱ����IAA���ȷֲ���һ��ʵ���Ʋ�CPT1�����ڸ���Ҳ��_____________��
��3���ۺ�����ʵ�����Ʋ⣬��Ca2+�ź������£�����������µ�ˮ�����ڵ�IAAͨ��_______������IAA�������뱳���ֲ������ȣ����ڸ���IAAŨ��________��ʹ������������ٶȱ���Ϊ________����˱��ֳ�������ԡ�