��Ŀ����
(9�� ÿ��1��)��ͼ�Ƕ�ֲ��ϸ�������ṹģʽͼ�����ͼ������?w_w w. k#s5_u.c o*m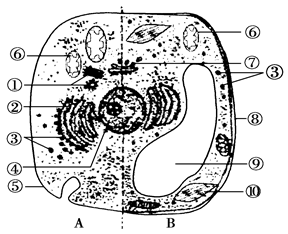
��1�� �Ƚ϶�ֲ��ϸ�������ṹ���ߵ�ֲ��ϸ���ڲ����е�ϸ�����ǣ� ��_________��
��2�� ��2������ϸ���ܹ���ȡ�����Ĵ��������ù��������ˢݵ�__________________�ԡ�
��3����B������ʾ��ϸ��Ϊ��и��������ϸ������Ӧ���еĽṹ��[ ] _________��[ ] _________��w_w+w.k_s_5*u.c_o*m
��4���ܶԵ����ʽ��мӹ��������ϸ�����ǣ� ��_________�ͣ� ��_________��w_w+w.k_s_5*u.c_o*m
��5��ԭ��ϸ����A������ʾϸ����ȣ���ṹ����Ҫ������___________________________��
��6����B������ʾ��ϸ��ΪҶ��ϸ���У��ṹ���в���һ��CO2���ӽ���ͬһ��ϸ���ڵĢ��б����ã�������Ҫ����_________��Ĥ,д���������������ܷ�Ӧʽ____________________��
(9��ÿ��1��) ��1���� ������ ��2��ϸ��Ĥ������ ��3�� [9]Һ�� [10]Ҷ���� ��4�� �� ������ �߸߶����� ��5�� ��ԭ��ϸ��û�к� Ĥ ��6�� 4
Ĥ ��6�� 4
CO2+H2O ��CH2O��+O2
����

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д���ÿ��1�֣���7�֣���ͼ��ʾֲ�ﲻͬ���ٶ������صķ�Ӧ�����ͼ�ش�

��1��A������Ӧ��������Ũ�ȶԾ�������ЧӦ�� ����ѿ������ЧӦ�� ��
��2��B������Ӧ��������Ũ�ȶԾ�������ЧӦ�� ��
��3����ͼ�п��Կ������������õ��ص�������������������������
��4��ij������ȤС�����ͼʾ��չ�����ᣨ������������ٽ�ij��ֲ���������������Ũ�ȵ�̽��ʵ�飬�����±����Ũ���ݶȡ�����λ��c/mol��L-1��
|
��� |
A |
B |
C |
|
Ũ�� |
10��12 |
10��9 |
10��7 |
Ϊ��һ����ȷ�ⶨ����Ũ�ȣ�Ӧ��ȡ�ĸĽ���ʩ���� ��
��5��ˮƽ���õĸ����������������������������˷�����ֲ�����������������λ���������صķֲ���ͬ�⣬�ڽ��ز�ͬʱҲ�����˴�������ϩ���ɴ˿�֪��ֲ������������������� �Ľ����
��6����������о�������ʱ�������м�˵���ѿ�ʣ�a���ͳ�ȥ��˵���ѿ�ʣ�b�����ڵ�����£����a�����Դ������b������Ҳ����������ʵ��Ľ����� ��

 ��6������ũ����̬ϵͳ�д�������ͼ��ʾ��ʳ��������E��Ⱥͬ����������Ϊ5.8��109ǧ����
��6������ũ����̬ϵͳ�д�������ͼ��ʾ��ʳ��������E��Ⱥͬ����������Ϊ5.8��109ǧ���� ��6������ũ����̬ϵͳ�д�������ͼ��ʾ��ʳ��������E��Ⱥͬ����������Ϊ5.8��109ǧ����
��6������ũ����̬ϵͳ�д�������ͼ��ʾ��ʳ��������E��Ⱥͬ����������Ϊ5.8��109ǧ����