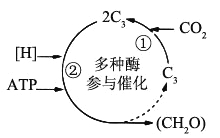
��Ŀ����
����Ŀ����ͼ������ƻ��������֭�����ƺ��Ĵ��¹������̣���ش��������⣺

��1����ƻ���ӹ��ɹ�֭�Ĺ����У���Ҫ�������ø������ø���Խ������ֽ��________________��ʹ���ǵĹ�֭��ó��塣Ϊ��̽���¶ȶԹ���ø���Ե�Ӱ�죬ͨ�������˳���ƻ��֭�� _______________ ���жϹ���ø���Եĸߵ͡�
��2��Ϊ�˽��й��Ƶķ���������ͨ���ȷ��봿����ĸ����Ȼ�������������̶�����ĸϸ�������Ž��֡����͡�
���ڷ��봿����ĸ��ʱ����Ҫ���������������ж�������ͨ�����ø��������
����ʹ��Һ�����������������Ĺ����У���Ҫ���ϵؽ�������裬Ŀ����____________�������ڽ�ĸ���ķ�ֳ��
���Ʊ��̶�����ĸϸ����ϸ�������____________���̶�������ĸϸ���̶�ǰ��Ҫ���л��ԭ����____________�������������������ɫ��dz���ʰ�ɫ��˵����������____________���̶��Ľ�ĸϸ����Ŀ______��
���ù̶�����ĸϸ�����͡�
��3��ƻ���ƾ�����һ�����Ϳ��γ�ƻ���ף��ڴ˹����У�Ҫ��ʱ��Һ��ͨ�������ԭ����____________��
���𰸡�������ȩ�� �����С �����ܽ���������ͬʱ�����ڽ�ĸ����Ӫ�����ʵij�ֽӴ� ���� ��ȱˮ״̬�����ﴦ������״̬��������ô�������״̬�Ľ�ĸ��ϸ���ָ�����������״̬ Ũ��ƫ�� ���� ������Ǻ���ϸ������������Ҫ����
��������
������ֲ��ϸ���ڵ���Ҫ�ɷ֣��������Ž�ֲ��ϸ��ճ����һ������ã�ȥ���������ͻ�ʹֲ����֯�����ɢ�������������Ҵ����Ǽ��������һ�ּ�������
��֭�ڽ�ĸ���������¾����ƾ����ͻ�ù��ƣ������ڴ��˾��������¾����������ͻ�ù��ס�
��1��������ֲ��ϸ���ڵ���Ҫ��ɳɷ֣������ɰ�����ȩ��Ͱ�����ȩ��������ɣ�����ø�����ƻ�ֲ��ϸ��������߳�֭�ʣ��ʿ�ͨ���ж��˳�ƻ��֭�������С�жϹ���ø�Ļ���ǿ����
��2�������ڽ�ĸ��Ϊ��������ϸ�����������������´�����ֳ��������������������е��ܽ���������Ҳ�����ڽ�ĸ����Ӫ�����ʳ�ֽӴ�������ʹ��Һ�����������������Ĺ����У���Ҫ���ϵؽ�������裻
��ϸ���̶�ͨ��ʹ�ð�����ĸ����ȱˮ״̬�´�������״̬�������ã�����Ҫ�ڹ̶�ǰ���лʹ֮�ָ�����������״̬�������������������ɫ��dz���ʰ�ɫ��˵����������Ũ��ƫ�ͣ��̶��Ľ�ĸϸ����Ŀ���٣�
��3��ƻ���Ʒ��ͳ�ƻ���Ĺ�����ͨ�����˾�����������ʵ�ֵģ����˾�Ϊ���������ڷ���������Ҫ������

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ���±��мס��ҷֱ��Ƿ���������������������������䷽��ͼ����ʾΪ��ط�����̡���ش��������⣨ע��ˮ�����أ���ˮ���ҵ��ף����ҵ���ˮ��õ�����
KH2PO4 | 1.4g | ��ά�ط� | 5.0g |
Na2HPO4 | 2.1g | NaNO3 | 1.0g |
MgSO4��7H2O | 0.2g | Na2HPO4 | 1.2g |
������ | 10.0g | KH2PO4 | 0.9g |
���� | 1.0g | MgSO4��7H2O | 0.5g |
��֬ | 15.0g | KCl | 0.5g |
��ĸ�� | 0.5g | ||
ˮ������ | 0.5g | ||
�����������ܽ��������ˮ���ݵ�1000mL | �����������ܽ��������ˮ���ݵ�1000mL | ||
�� | �� | ||

��1�������������ṩ��Դ��������________��
��2������������������Ƴɹ��������������ڸ��������ϳ����ľ����Ƿ��ֽܷ���ά�أ�________����ǡ���������������м���________����ɸѡ�����зֽ���ά�������ľ��꣬ͬʱ��ȦԽ________�����С��������ʾ�þ��ֽ���ά�ص�����Խǿ��
��3��Ϊ�˼��������������ͼ������_______�����н�����������������֮�⣬�����Բ���_______�����м���������ǰһ�ּ����������ý��Ҫ�Ⱥ�һ�ּ����������ý��_____�����С������
��4��һλͬѧ����ͼ���ķ���������4��ƽ���������Ϸֱ����0.1mL����ϡ��Һ�ܣ�������������ֱ�Ϊ180��155��176��129������ÿ��������Ʒ����������ԼΪ________________����