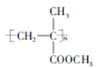��Ŀ����
����Ŀ���������ʼ��仯���ﳣӦ�����л���ķ�Ӧ�ͷ���֮�У�ij�����廯����A����ʽΪC8H10O2 ,Ϊ�ⶨ��ṹ�����·�����
��1��Ϊȷ���ǻ��ĸ����� ��1mol A�������Ʒ�Ӧ��������22.4L(��״����)��˵��A�����к��ǻ�________����
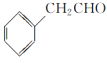
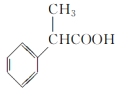
��2���˴Ź���������ʾA��3���壬�����֮��Ϊ1��2��2�������ʵĽṹ��ʽΪ______________________��
��3��A��Cu���¿ɱ��������������л���B��B����Է���������AС4����д����Ӧ�ķ���ʽ_________________________________________________��
��4��1mol B������������Һ��ַ�Ӧ�����л���C��ͬʱ�õ���_______�ˡ���д����ѧ��Ӧ����ʽ_______________________________________________�� (ԭ������Ag����108)
��5���л���F���л���B��һ��ͬ���칹�塣1mol F�������Ʒ�Ӧͬ����������22.4L(��״����)����F��ʹ�Ȼ�����Һ����ɫ����д��������������л���F�Ľṹ��ʽ______________________(ֻд��һ�ּ���)��
���𰸡�
��1��2
��2��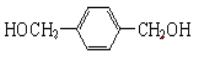
��3�� 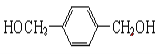 + O2
+ O2![]()
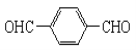 +2H2O
+2H2O
��4�� 432
![]()
��5�� 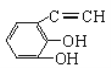
��������
�����������1����״����22.4L���������ʵ���Ϊ![]() =1mol������1mol������Ҫ����2mol-OH��A�����к����ǻ�����ĿΪ
=1mol������1mol������Ҫ����2mol-OH��A�����к����ǻ�����ĿΪ![]() =2��
=2��
��2�������廯����A����ʽΪC8H10O2���䲻���Ͷ�Ϊ![]() =4��1�������IJ����Ͷ�Ϊ4��˵��A�����в��������������ͽṹ���˴Ź���H����ʾA��3���壬�����֮��Ϊ1��2��2��˵��A���Ӿ��жԳƽṹ���ڱ����Ķ�λC�ϸ�����1��-CH2����A�Ľṹ��ʽΪ��
=4��1�������IJ����Ͷ�Ϊ4��˵��A�����в��������������ͽṹ���˴Ź���H����ʾA��3���壬�����֮��Ϊ1��2��2��˵��A���Ӿ��жԳƽṹ���ڱ����Ķ�λC�ϸ�����1��-CH2����A�Ľṹ��ʽΪ��![]() ��
��
��3��A�Ľṹ��ʽΪ![]() ��A��Cu���¿ɱ��������������л���B��B����Է���������AС4����B�����к�������ȩ����B�Ľṹ��ʽΪ��
��A��Cu���¿ɱ��������������л���B��B����Է���������AС4����B�����к�������ȩ����B�Ľṹ��ʽΪ��![]() ���÷�Ӧ�Ļ�ѧ����ʽΪ��
���÷�Ӧ�Ļ�ѧ����ʽΪ��![]() +O2
+O2![]()
![]() +2H2O��
+2H2O��
��4��1mol B(![]() )������������Һ��ַ�Ӧ�����л���C����C�Ľṹ��ʽΪ
)������������Һ��ַ�Ӧ�����л���C����C�Ľṹ��ʽΪ![]() �����ݹ�ϵʽ-HCO��2Ag��֪��1molB�����к���2molȩ�����ܹ��û���4molAg���û�������������Ϊ��108g/mol��4mol=432g����Ӧ�ķ���ʽΪ
�����ݹ�ϵʽ-HCO��2Ag��֪��1molB�����к���2molȩ�����ܹ��û���4molAg���û�������������Ϊ��108g/mol��4mol=432g����Ӧ�ķ���ʽΪ![]() ��
��
��5��BΪ![]() ���л���F���л���B��һ��ͬ���칹�壮1mol F�������Ʒ�Ӧͬ����������22.4L��˵��F����2���ǻ�����Ӧ��C��C�������Ӧ��ͬ���칹��Ϊ
���л���F���л���B��һ��ͬ���칹�壮1mol F�������Ʒ�Ӧͬ����������22.4L��˵��F����2���ǻ�����Ӧ��C��C�������Ӧ��ͬ���칹��Ϊ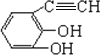 ����
����