��Ŀ����
��������е���Ϣ�ش����⡣
���������ͼ�е���Ϣ�ش�
��1���������ڵ�ϸ����Һ����ѹ����ʱ�� ���ڵIJ��ɴ����Ҷ�ͷŵ���ͼ�м���X�� �ĺ������ӣ�ʹ�����ļ��٣�ͬʱ �����ʸУ�ʹ��������ˮ���Ӷ�ʹϸ����Һ����ѹ�ָ�������
���ӣ�
��2����ͼ������״�ټ��صķּ������д��� ���ơ�
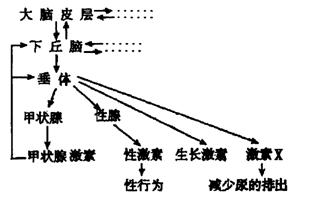
���±��Dz�ͬŨ�ȵ��Ͳ�������ˮ��Һ���۲���������Ӱ���ʵ����������Ũ��a��Ũ��e�ɵ͵������У�����ش��������⣺
| ��� | ��ˮ | Ũ��a | Ũ��b | Ũ��c | Ũ��d | Ũ��e |
| ƽ����ߣ�cm�� | 16 | 20 | 38 | 51 | 42 | 24 |
��1����ʵ�����ܷ�˵���Ͳ����������۲��������������ԣ� ��
������ ��
��2�������ƽ�һ��̽���Ͳ��������ٽ��۲�����������Ũ�ȷ�Χ��ʵ�鲽�裺
�� ��
��ȡͬһ������ʹ���ȷ�������ѡȡ��ߡ�������ͬ���۲����� ��
�۷ֱ� ������Ӧ����۲����磻
������ͬ�����˵�����������һ��ʱ�䣬 ��
��1����������ϸ����1�֣� �������أ�1�֣� ����Ƥ�㣨1�֣�
��2���������ڣ�1�֣�
��1����1�֣�
��ʵ����ֻ����a~e����Ũ�Ⱦ��дٽ����ã�û������������
��2������Ũ��b��Ũ��d֮������ϵ��Ũ���ݶȵ��Ͳ�������ˮ��Һ
�ھ���Ϊ4�飨��4�����ϣ���ÿ��30�꣬���
���õ���������������ϵ��Ũ�ȵ��Ͳ�������ˮ��Һ
�ܲ�������¼�۲���ߣ��ټ���ƽ��ֵ
����:
��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д���������е���Ϣ�ش����⡣
���������ͼ�е���Ϣ�ش�
��1���������ڵ�ϸ����Һ����ѹ����ʱ�� ���ڵIJ��ɴ����Ҷ�ͷŵ���ͼ�м���X��  �ĺ������ӣ�ʹ�����ļ��٣�ͬʱ �����ʸУ�ʹ��������ˮ���Ӷ�ʹϸ����Һ����ѹ�ָ�������
�ĺ������ӣ�ʹ�����ļ��٣�ͬʱ �����ʸУ�ʹ��������ˮ���Ӷ�ʹϸ����Һ����ѹ�ָ�������
���ӣ�
��2����ͼ������״�ټ��صķּ������д��� ���ơ�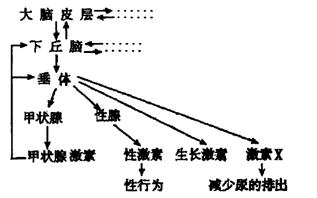
���±��Dz�ͬŨ�ȵ��Ͳ�������ˮ��Һ���۲���������Ӱ���ʵ����������Ũ��a��Ũ��e�ɵ͵������У�����ش��������⣺
| ��� | ��ˮ | Ũ��a | Ũ��b | Ũ��c | Ũ��d | Ũ��e |
| ƽ����ߣ�cm�� | 16 | 20 | 38 | 51 | 42 | 24 |
������ ��
��2�������ƽ�һ��̽���Ͳ��������ٽ��۲�����������Ũ�ȷ�Χ��ʵ�鲽�裺
�� ��
��ȡͬһ������ʹ���ȷ�������ѡȡ��ߡ�������ͬ���۲����� ��
�۷ֱ� ������Ӧ����۲����磻
������ͬ�����˵�����������һ��ʱ�䣬 ��
��������е���Ϣ�ش����⡣
���������ͼ�е���Ϣ�ش�
��1���������ڵ�ϸ����Һ����ѹ����ʱ�� ���ڵIJ��ɴ����Ҷ�ͷŵ���ͼ�м���X�� �ĺ������ӣ�ʹ�����ļ��٣�ͬʱ �����ʸУ�ʹ��������ˮ���Ӷ�ʹϸ����Һ����ѹ�ָ�������
���ӣ�
��2����ͼ������״�ټ��صķּ������д��� ���ơ�
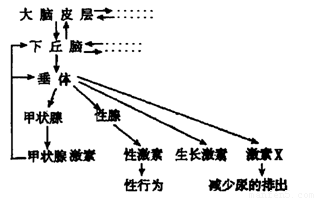
���±��Dz�ͬŨ�ȵ��Ͳ�������ˮ��Һ���۲���������Ӱ���ʵ����������Ũ��a��Ũ��e�ɵ͵������У�����ش��������⣺
|
��� |
��ˮ |
Ũ��a |
Ũ��b |
Ũ��c |
Ũ��d |
Ũ��e |
|
ƽ����ߣ�cm�� |
16 |
20 |
38 |
51 |
42 |
24 |
��1����ʵ�����ܷ�˵���Ͳ����������۲��������������ԣ� ��
������ ��
��2�������ƽ�һ��̽���Ͳ��������ٽ��۲�����������Ũ�ȷ�Χ��ʵ�鲽�裺
�� ��
��ȡͬһ������ʹ���ȷ�������ѡȡ��ߡ�������ͬ���۲����� ��
�۷ֱ� ������Ӧ����۲����磻
������ͬ�����˵�����������һ��ʱ�䣬 ��
��12�֣���������е���Ϣ�ش����⡣
I����3�֣�Ϊ̽������Һ�н�ĸ����Ⱥ�����Ķ�̬�仯��ij��ȤС�鰴�±�������й�ʵ�鲢���ڶ�����Һ�еĽ�ĸ�����м��������ݶ�μ�����ƽ��ֵ���Ƴ���ĸ��ϸ����Ŀ�仯����ͼ��������ͼ������Ϊ��ĸ��ϸ����������ش�
|
�Թܱ�� |
����Һ��mL |
��ĸ����mL |
�����¶ȣ��棩 |
|
A1��A2��A3 |
10 |
0.1 |
20 |
|
B1��B2��B3 |
10 |
0.1 |
10 |
|
C1��C2��C3 |
10 |
0.1 |
5 |
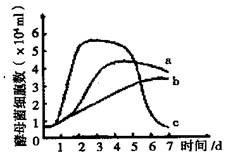
��С��̽���Ŀ����� ����ʵ������д��ڵIJ���֮���� ��ͼ������ܴ���A���ĸ����Ⱥ�����仯�������� ��
II����9�֣��±��Dz�ͬŨ�ȵ��Ͳ�������ˮ��Һ���۲���������Ӱ���ʵ����
|
��� |
��ˮ |
Ũ��a |
Ũ��b |
Ũ��c |
Ũ��d |
Ũ��e |
|
ƽ����ߣ�cm�� |
16 |
20 |
38 |
51 |
42 |
24 |
��1����ʵ�����ܷ�˵���Ͳ����������۲��������������ԣ� �������� ��
��2�������ƽ�һ��̽���Ͳ��������ٽ��۲�����������Ũ�ȷ�Χ��ʵ�鲽�裺
�� ��
��ȡͬһ������ʹ���ȷ�������ѡȡ��ߡ�������ͬ���۲����� ��
�۷ֱ� ������Ӧ����۲����磻
������ͬ�����˵�����������һ��ʱ��� ���ټ���ƽ��ֵ��
