��Ŀ����
����Ŀ����ͼ�ǹ��ƺ���������ʵ�����̺�ijͬѧ��ƵĹ��ƺ��ķ���װ�á�����ͼʾ�ش��������⣺
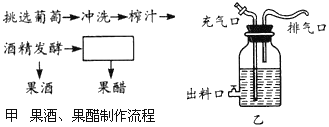
(1)���ͼ���е�ʵ�����̡�_________
(2)��ϴ����ҪĿ����_______����ϴӦ�ر�ע�ⲻ��________���Է�ֹ���ֵ���ʧ��
(3)ͼ��װ���еij�������_________ʱ�رգ���______ʱ���ӳ����ã����������ϵ�����________��
(4)�������ڹ��Ʒ���ʱ�ų�����������_______������_______
(5)д������ơ��������йصķ�Ӧ����ʽ��__________
(6)�ھƾ�����ʱƿ���¶�һ��Ӧ������___________
���𰸡����ᷢ�� ϴȥ���� ������ϴ ���Ʒ��� ���� �������� ��ĸ�� CO2 C6H12O6![]() 2CO2+2C2H5OH+������C6H12O6+O2
2CO2+2C2H5OH+������C6H12O6+O2![]() CH3COOH+CO2+H2O��C2H5OH+O2
CH3COOH+CO2+H2O��C2H5OH+O2![]() CH3COOH+H2O�� 20��
CH3COOH+H2O�� 20��
��������
����ͼ�ף�ͼ���ǹ��ƺ���������ʵ�����̣��ƾ����ͺ��Խ��й����͡�
����ͼ�ң�ͼ���ǹ��ƺ��ķ���װ��ͼ�����г��Ͽڵ������dz��ϡ���⣻�����ڵ��������ڹ�������ʱͨ�������ģ����������ų��������в��������壬�����������ĺô��Ƿ�ֹ�Ӿ���������Ⱦ��
��1�������Ϸ�����֪������Ϊ���ᷢ�͡�
��2����ϴ����ҪĿ����ϴȥˮ������ĸ��������ڷ��͵ľ�������ˮ�������ϵ�Ұ�����֣��ʳ�ϴӦ�ر�ע�ⲻ�ܷ�����ϴ���Է�ֹ���ֵ���ʧ��
��3������������Ҫ�������������ͼ��װ���еij������ڹ��Ʒ�������Ҫ�رա����ڴ����Ϊ�����������ڹ�����ʱ��������Ӧ���ӳ����ã����������ϵ����ڱ���������
��4�����Ʒ������г��˲����ƾ��������ͷŴ���������̼������������ڸù�����Ӧʱ����Ŀ���������ų���ĸ���������������Ķ�����̼��
��5�����Ʒ��͵ķ�ӦʽΪ��C6H12O6![]() 2CO2+2C2H5OH+�����������͵ķ�ӦʽΪ��C6H12O6+O2
2CO2+2C2H5OH+�����������͵ķ�ӦʽΪ��C6H12O6+O2![]() CH3COOH+CO2+H2O��C2H5OH+O2
CH3COOH+CO2+H2O��C2H5OH+O2![]() CH3COOH+H2O��
CH3COOH+H2O��
��6���ھƾ�����ʱƿ���¶�һ��Ӧ������20�档

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�