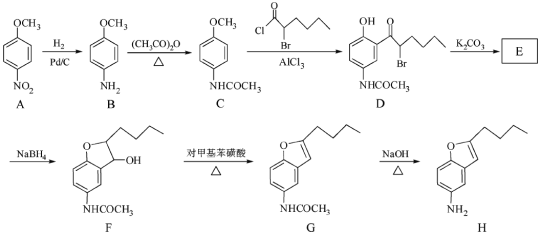
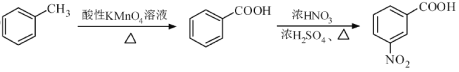
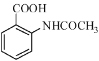
��Ŀ����
����Ŀ���������ʱڷ�����ʱڷ��븴ԭ�ķ����ⶨ��ɫ������Ҷ��Ƥϸ����Ũ��ʵ���У�����ɫ�������Ҷ��Ƥϸ���ֱ���벻ͬŨ�ȣ�A��F�����������Һ�С�5min�۲��ҶƬ��Ƥϸ�����ʱڷ��뼰��ԭ��ʵ�������£�
ҶƬ��Ƥϸ�� | A | B | C | D | E | F |
�������Һ/ ��mol��L��1�� | 0��11 | 0��12 | 0��125 | 0��13 | 0��14 | 0��50 |
�ʱڷ���̶� | δ���� | δ���� | ��ʼ���� | ���� | �������� | �������� |
�ʱڷ��븴ԭ | δ��ԭ | δ��ԭ | �Զ���ԭ | �Զ���ԭ | ���յ���ԭ | ����ԭ |
����ʵ�����������ش��������⣺
��1���ʱڷ���ָ����___________��������ڸ�ʵ���У��ʱ�֮���������_________��
��2����C��D��Ũ���£�ҶƬ��Ƥϸ���������ʱڷ�����Զ���ԭ��ԭ����______________��
��3����FŨ���£���ҶƬϸ�������ʱڷ��������ˮ����ʹ�临ԭ��ԭ����______________��
��4�����ݸ�ʵ������������ҶƬ��Ƥϸ����Ũ��Լ��____________֮�䡣
���𰸡�ϸ���ں�ԭ���ʲ���� �������Һ ԭ���ʲ����ѡ�����ԣ�K����NO3-�����䵽ϸ���ڲ���ʹϸ��Һ��Ũ�����ߣ�ˮ����������ɢ����ϸ���������ʱڷ��븴ԭ �ڸ�Ũ����Һ�У�ϸ����ʧˮ�����������ԭ���ʲ�ʧȥѡ������ 0.12��0.125mol/L
��������
��ϸ��Һ��Ũ��С�������Һ��Ũ��ʱ��ϸ��Һ�е�ˮ�־���ԭ���ʲ���뵽�����Һ�У�����ԭ���ʲ��ϸ���ڵ������Դ�ϸ������ʧˮʱ��Һ������С��ԭ���ʲ�ͻ���ϸ�������뿪�������������ʱڷ��롣��ϸ��Һ��Ũ�ȴ��������Һ��Ũ��ʱ�������Һ�е�ˮ�־���ԭ���ʲ���뵽ϸ��Һ�У�Һ���������ԭ���ʲ�ͻ������ػָ���ԭ����״̬�����������ʱڷ��븴ԭ����ȷ֪ʶ�㣬������صĻ���֪ʶ������ͼ�����������ľ�����ʾ�ۺ�����
��1���ʱڷ���ָ����ϸ������ԭ���ʲ���룬����ϸ���ھ���ȫ�ԣ���������ʱ�֮����������������Һ��
��2������K+��NO3-ͨ��������������Һ�ݣ�ʹϸ��ҺŨ������������ϸ��Һ��Ũ�ȴ��������Һ��Ũ�ȣ�ҶƬ��Ƥϸ����ˮ���Ӷ������ʱڷ�����Զ���ԭ��
��3������FŨ�ȹ��ߣ�ϸ��ʧˮ���������������ҶƬϸ�������ʱڷ��������ˮ������ʹ�临ԭ��
��4��ϸ������0��12mol/L��KNO3��Һ�У��������ʱڷ��룬˵��ϸ��ҺŨ�ȴ���0��12mol/L��ϸ������0��125mol/L��KNO3��Һ�У������ʱڷ��룬˵��ϸ��ҺŨ��С��0��125mol/L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�