��Ŀ����
������������Ķ��Ļ��������������һ���Ȱ��ᡢһ�����װ��ᡢһ���ʰ��ṹ�ɵ����Ļ��������ӽṹ����ͼ��ʾ�����ձ�����ڶ�ֲ��ϸ��������ϸ���С��ڸ÷�������һ������Ħã��ļ������й��ڸ����Ļ�����������У���ȷ����(����)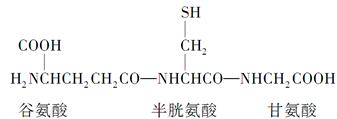
| A���ڹ����ķ����к��������ļ� |
| B���ã��ļ��ڹȰ���Ͱ��װ���֮�� |
| C���ã��ļ��ڰ��װ���ʰ���֮�� |
| D���÷����к���һ��������һ���Ȼ� |
B
��������������ã��ļ����ɹȰ�����Ӧ�λ̼ԭ���ϵ��Ȼ������γɡ�
���㣺���������ˮ����
���������⿼��ѧ���Ի���֪ʶ�����գ������������ͣ�����ѧ������������������ݡ�

��ϰ��ϵ�д�
�����Ŀ
 ��������֤ʵ����������Ĺ�ϵ�Ŀ�ѧ���� ���������ʵ���������� ����ѧʵ�鷽�����ƣ��ŵó����ۡ�
��������֤ʵ����������Ĺ�ϵ�Ŀ�ѧ���� ���������ʵ���������� ����ѧʵ�鷽�����ƣ��ŵó����ۡ� �Ƚ����ƽ�û���ձ��� �У��۲��Ƿ�����ѣ������� ���ص㣬���������� ���۲쵰���Ƿ�����ѣ���������ѣ�˵����Ĥ�к��е����ʷ��ӡ��Դ����ƣ��������Ʋ���Ĥ�������ɷ֡�
�Ƚ����ƽ�û���ձ��� �У��۲��Ƿ�����ѣ������� ���ص㣬���������� ���۲쵰���Ƿ�����ѣ���������ѣ�˵����Ĥ�к��е����ʷ��ӡ��Դ����ƣ��������Ʋ���Ĥ�������ɷ֡� �裺
�裺 �У���Ȼ������37��ĺ����䣨���߾���30���������ϵ����»������С�
�У���Ȼ������37��ĺ����䣨���߾���30���������ϵ����»������С� �շ����Ʋ����Ĥ�ijɷ֡�
�շ����Ʋ����Ĥ�ijɷ֡�