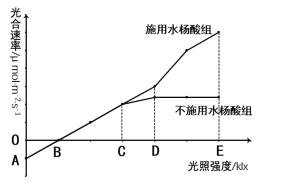
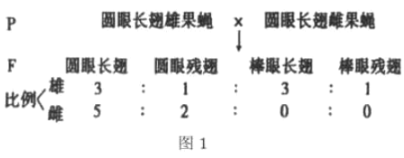
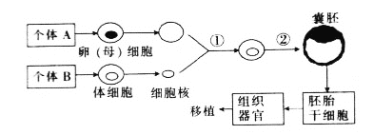
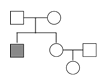
��Ŀ����
����Ŀ���߷���H��һ�ֳ�Ĥ���õ���֬����ϳ�·�����£�
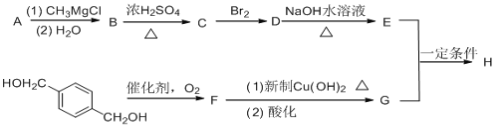
��֪����A����Է�������Ϊ58����Ԫ����������Ϊ0.276���˴Ź���������ʾֻ��һ��壻
��![]()
��1��A�Ľṹ��ʽΪ___��G�й���������Ϊ___��
��2����B����C�Ļ�ѧ����ʽΪ___��
��3��B��ϵͳ����Ϊ___��
��4��������E�ķе�___(ѡ����>������<��������=��)2-�����顣
��5��F������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽΪ___��
��6��H�Ľṹ��ʽΪ___��
��7��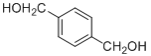 �ķ�������������ͬ���칹����__��
�ķ�������������ͬ���칹����__��
�ٷ�������״�ṹ����֧���ں˴Ź�������������壬�����֮��Ϊ3��2�����й���������H2�����ӳɷ�Ӧ���л���ṹ��ʽΪ___(��дһ��)��
���𰸡�![]() �Ȼ�
�Ȼ� 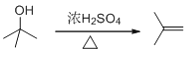 +H2O 2-��-2-���� >
+H2O 2-��-2-���� > 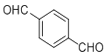 +Cu(OH)2+2NaOH
+Cu(OH)2+2NaOH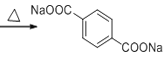 +2Cu2O��+6H2O
+2Cu2O��+6H2O 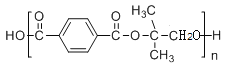 8
8 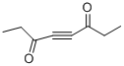 ��
��
��������
A����Է�������Ϊ58����Ԫ����������Ϊ0.276��������Ԫ�صĸ���Ϊ![]() ����A�к���һ����ԭ�ӣ�ʣ��ΪC��HԪ�أ�������Է���������ϵ�ó�����ʽΪC2H6O������Ϊ�˴Ź���������ʾֻ��һ��壬����A�Ľṹ��Ϊ
����A�к���һ����ԭ�ӣ�ʣ��ΪC��HԪ�أ�������Է���������ϵ�ó�����ʽΪC2H6O������Ϊ�˴Ź���������ʾֻ��һ��壬����A�Ľṹ��Ϊ![]() ��������֪���Ƴ�B�ĽṹΪ
��������֪���Ƴ�B�ĽṹΪ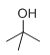 ������Ũ������������·�����ȥ��Ӧ����ϩ������C�ĽṹΪ
������Ũ������������·�����ȥ��Ӧ����ϩ������C�ĽṹΪ![]() ��ϩ�����嵥�ʷ����ӳɷ�Ӧ����±��������D�ĽṹΪ
��ϩ�����嵥�ʷ����ӳɷ�Ӧ����±��������D�ĽṹΪ![]() ��D����������ˮ��Һ���������·���ˮ������E����E�ĽṹΪ
��D����������ˮ��Һ���������·���ˮ������E����E�ĽṹΪ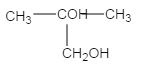 ��
��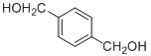 �ڴ��������������·������Ĵ���������ȩ����F�ĽṹΪ
�ڴ��������������·������Ĵ���������ȩ����F�ĽṹΪ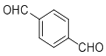 ��ȫ������������ͭ��Һ�м��Ȳ��ữ�������ᣬ��G�ĽṹΪ
��ȫ������������ͭ��Һ�м��Ȳ��ữ�������ᣬ��G�ĽṹΪ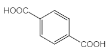 ��G��E������������H��
��G��E������������H��
(1)���ɷ�����֪A�ĽṹΪ![]() ��G�й���������Ϊ�Ȼ���
��G�й���������Ϊ�Ȼ���
(2)����B����C�Ļ�ѧ����ʽΪ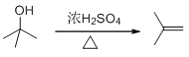 +H2O��
+H2O��
(3)��B�ĽṹΪ ������ϵͳ����������B������Ϊ2-��-2-������
������ϵͳ����������B������Ϊ2-��-2-������
(4)��������E�ĽṹΪ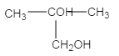 ����Է�����������2-�������������۷е���ߣ��ʴ�Ϊ>��
����Է�����������2-�������������۷е���ߣ��ʴ�Ϊ>��
(5)��F������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽΪ��
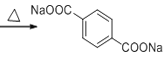 +2Cu2O��+6H2O��
+2Cu2O��+6H2O��
(6)��H��G��E�������γɣ��ʻ�ѧʽΪ��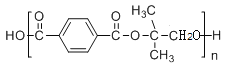 ��
��
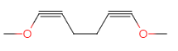
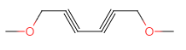
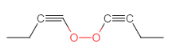
(7)�� ��������״�ṹ����֧���������ĸ�˫����һ����������˫���������������Һ˴Ź�������������壬�����֮��Ϊ3��2������Ҫ��Ľṹ�У�
��������״�ṹ����֧���������ĸ�˫����һ����������˫���������������Һ˴Ź�������������壬�����֮��Ϊ3��2������Ҫ��Ľṹ�У�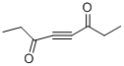 ��
�� ��
�� ��
�� ��
��![]() ��
��![]() ��
�� ��
��![]() ���ʴ�Ϊ��8��
���ʴ�Ϊ��8�� ��
�� ��
��