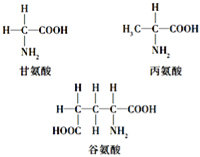
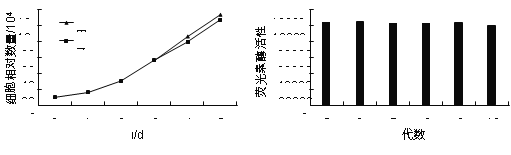
��Ŀ����
����Ŀ��ũ�����ݲ˵����������������ʵ��߲˾��������������볹����ϴ���ðƲ��ù����ݲ�̳�У��ݲ�̳һ������ͷС�м���������̳����̳�أ������ѷ�IJ�̳�����á�Ȼ�������ˮ�������ϼ�һЩ�����ݲ�ˮ�����ܷ�����������������ʻ����¶�Ϊ28��32 �档��ʱ�������ݲ˻ᡰ�̶����ᡱ��������̡���ǰ�������ι��࣬���������ι��١�
(1)��ˮ����в���ȴ��ſ�ʹ�ã����������Ϊ��__________________________________________����ȴ֮��ʹ����Ϊ�˱�֤���������������������Ӱ�졣
(2)��̳�ܷ��ԭ���DZ�֤��������������______________���߲˸���̳ʱ���������в�����Ĵ˾��ͽ�ĸ����������еĽ�ĸ������ĺ������÷�ʽ��__________��
��д����ط�Ӧʽ____________________________________��
(3)����һЩ�����ݲ�ˮ����������____________________________________________��
(4)�����ݲ˵Ĺ����У��л���ĸ���_________(����ӡ����١��� ����̳���л��������__________(����ӡ����١�����
(5)�ⶨ�������κ����ķ�����__________����ԭ���ǣ��������ữ�����£�������������������ᷢ���ص�����Ӧ����N��1�������Ҷ��������ν���γ�___________________������ɫ��Ӧ�����Ʒ����֪Ũ�ȵı���ɫҺ����Ŀ��Ƚϣ����Դ��¹�����ݲ����������εĺ�����
���𰸡���ȥˮ�е�������ɱ����ˮ�е��Ӿ� �������� �������� C6H12O6��6O2��6H2O![]() 6CO2��12H2O������ �ṩ��������֣�ʹ���ڶ�ʱ�����γɾ������� ���� ���� ��ɫ�� õ���ɫȾ��
6CO2��12H2O������ �ṩ��������֣�ʹ���ڶ�ʱ�����γɾ������� ���� ���� ��ɫ�� õ���ɫȾ��
��������
�ݲ�������ԭ����������������������������ᣬΪ�˷�ֹ������Ⱦ�������ݲ˹�����Ӧ�������������ݲ���ˮ��Ũ��Ӧ������ˮ���εı���ӦΪ4��1�������Ũ�ȹ���������������ͣ�����̶����ᣬ��Ũ�ȹ��Ͳ�����������������������Ӿ���Ⱦ��Ϊ����������ʱ����Լ�����ݲ�ˮ������������������������������õ����������ٰ���̼Դ����Դ��ˮ�����εȡ�
��1�������ݲ�ʱ����ˮ����в���ȴ��ʹ�ã����м�����е�Ŀ���dz�ȥˮ�е�������ɱ����ˮ�е��Ӿ���
��2������������������������̳�ܷ����Ϊ������ṩ������������ĸ������ĺ������÷�ʽ�������������䷴ӦʽΪ��C6H12O6��6O2��6H2O![]() 6CO2��12H2O��������
6CO2��12H2O��������
��3�������ݲ�ˮ���к��д���������������ṩ��������֣�ʹ���ڶ�ʱ�����γɾ������ƣ�����������ݲ˵�ʱ����Ҫ���������ݲ�ˮ����
��4�������������������л�����л���ת����������ʣ�����л���ĸ��ؼ��٣��л�����������ࡣ
��5���ⶨ�������κ����ķ����DZ�ɫ������ԭ���ǣ��������ữ�����£�������������������ᷢ���ص�����Ӧ����N��1�������Ҷ��������ν���γ�õ���ɫȾ�ϣ�����ɫ��Ӧ�����Ʒ����֪Ũ�ȵı���ɫҺ����Ŀ��Ƚϣ����Դ��¹�����ݲ����������εĺ�����