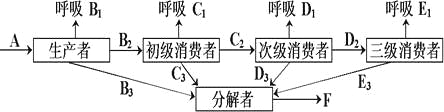
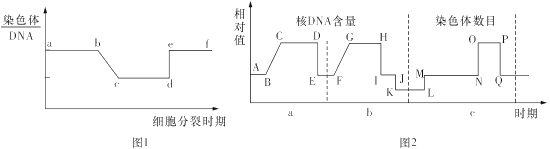
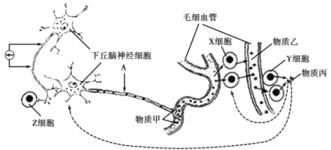
��Ŀ����
����Ŀ����ͼ�ǹ�����ù��̵�ͼ�⣬���ͼ��ϸ������ش��������⣺
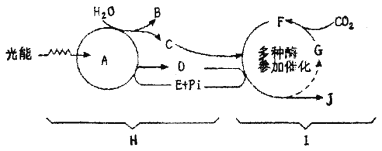
��1��ͼ��H���̱�ʾ___________________��I���̱�ʾ____________________��
��2��ͼ��A����______________��������������Ҫ��______________________��
��3��ͼ��B������_____________������ͨ��ҶƬ�����ṹ�ŵ������еġ�
��4��ͼ��C��Ҷ�е��γɲ�λ��_____________________�ϣ�������������________________��
��5��ͼ��D��������������������浽_____________����ͼ�е���ĸ����
��6�������һ���ڹ��µ�ֲ�ͻȻת�Ƶ��ڰ��ĵط�����ͼ����һ�����ʵ��������нϴ���ȵ�����__________________
A��F���������� B��D������������C��J������������D��G
��7����д��ͼ��E�Ľṹ��ʽ�� __________________________��
��8����д��������õ��ܷ�Ӧʽ��________________________��
���𰸡��ⷴӦ ����Ӧ Ҷ�����е�ɫ�� ���ա����ݺ�ת������ ˮ�Ĺ�� �����屡Ĥ �ڰ���Ӧ�л�ԭ��̼������ J A A-P��P 6CO2+12H2O![]() C6H12O6+6O2+6H2O
C6H12O6+6O2+6H2O
��������
����ͼʾ�����ݹⷴӦ�Ͱ���Ӧ֮��Ĺ�ϵ���ⷴӦΪ����Ӧ�ṩ[H]��ATP������ӦΪ�ⷴӦ�ṩADP��Pi�������ж�H�ǹⷴӦ��I�ǰ���Ӧ��A��Ҷ������ɫ�أ�B��O2��C��[H]��D��ATP��E��ADP��F����̼�����G����̼�����J�ǣ�CH2O����
��1���������Ϸ�����֪��ͼ��H���̱�ʾ�ⷴӦ��I���̱�ʾ����Ӧ��
��2���������Ϸ�����֪��ͼ��A����Ҷ������ɫ�أ�������������Ҫ�����ա����ݺ�ת�����ܡ�
��3��ͼ��B��O2��������ˮ�Ĺ�⣬��ͨ��ҶƬ�������ŵ�������ȥ��
��4��C��[H]���Ƿ����������屡Ĥ�ϹⷴӦ�����ģ����ڰ���Ӧ��������̼������Ļ�ԭ��
��5��ͼ��D������ATP�����ڰ���Ӧ����̼������Ļ�ԭ�����ATP�е�����������浽J�л������ˡ�
��6�������һ���ڹ��µ�ֲ�ͻȻת�Ƶ��ڰ��ĵط����ⷴӦ������[H]��ATP���٣�����Ӧ�б���ԭ��C3���٣���CO2��C5�̶��γ�C3�Ĺ��̲��䣬��ͼ��C3��F�����������нϴ���ȵ����ӣ���ѡA��
��7��ͼ��E��ADP����ṹ��ʽΪA-P��P��
��8��������õ��ܷ�ӦʽΪ��6CO2+12H2O![]() C6H12O6+6O2+6H2O��
C6H12O6+6O2+6H2O��

 ����������ϵ�д�
����������ϵ�д�