��Ŀ����
A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ��������ӻ�������ҡ�D��A��ԭ�Ӹ�����3��2�γ����ӻ��������E�ǵؿ��к�����ߵĽ���Ԫ�ء�����������Ϣ�ش��������⣺��ÿ��2�֣���8�֣�
��1��EԪ�������ڱ��е�λ����____________��
��2������A��ԭ�ӽṹ��ͼ
��3�����ӻ������ҵĵ���ʽ��
��4��C��D��E�γɵļ����Ӱ뾶�ɴ�С��˳���ǣ������ӷ��ű�ʾ��
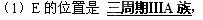

����A�DN��B�DO��C�DNa��D�DMg��E�DAl ���רD Na2O ���ҨD Na2O2 �����DMg3N2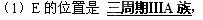
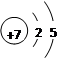 ��A��ԭ�ӽṹʾ��ͼ��
��A��ԭ�ӽṹʾ��ͼ��
��Na2O2�ĵ���ʽ��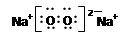
�ȵ��Ӳ�ṹ��ͬ�����ӣ��˵��Խ�뾶ԽС�����Ӱ뾶�ɴ�С��˳����Na�� Mg2�� Al3�� .

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ
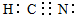
 CH3COOH+OH-
CH3COOH+OH- ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ�� ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��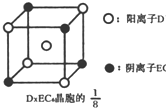
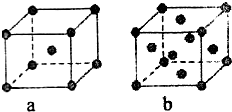 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��