��Ŀ����
�����ѣ�CH3OCH3������ɫ���壬����Ϊһ��������Դ���ɺϳ��������ΪH2��CO��������CO2��ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
��i��CO(g) + 2H2(g) = CH3OH(g) ��H1 = -90.1kJ?mol-1
��ii��CO2(g) + 3H2(g) = CH3OH(g) + H2O(g) ��H2 = -49.0kJ?mol-1
ˮú���任��Ӧ��
��iii��CO(g) + H2O(g) = CO2(g) + H2 (g) ��H3 = -41.1kJ?mol-1
�����Ѻϳɷ�Ӧ��
��iV��2 CH3OH(g) = CH3OCH3(g) + H2O(g) ��H4 = -24.5kJ?mol-1
�ش��������⣺
��1��Al2O3�Ǻϳ���ֱ���Ʊ������ѷ�Ӧ��������Ҫ�ɷ�֮һ����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ���������� ���Ի�ѧ����ʽ��ʾ����
��2�����������Ѻϳɷ�Ӧ��iV������COת���ʵ�Ӱ�� ��
��3����H2��COֱ���Ʊ������ѣ���һ����Ϊˮ���������Ȼ�ѧ����ʽΪ �����ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ�� ��
��4�����о����ڴ�������Cu��Zn��Al��O��Al2O3����ѹǿΪ5.0MPa�������£���H2��COֱ���Ʊ������ѣ��������ͼ��ʾ������COת�������¶����߶����͵�ԭ���� ��

��5��������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㣬�������ܶȵ��ڼ״�ֱ��ȼ�ϵ�أ�5.93kW?h?kg-1�����������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ ��һ�������ѷ��Ӿ����绯ѧ���������Բ��� �����ӵ��������õ�ص����������ѹΪ1.20V�������ܶ�E = ����ʽ���㡣�����ܶ�=����������/ȼ��������1 kW?h = 3.6��106J����
�״��ϳɷ�Ӧ��
��i��CO(g) + 2H2(g) = CH3OH(g) ��H1 = -90.1kJ?mol-1
��ii��CO2(g) + 3H2(g) = CH3OH(g) + H2O(g) ��H2 = -49.0kJ?mol-1
ˮú���任��Ӧ��
��iii��CO(g) + H2O(g) = CO2(g) + H2 (g) ��H3 = -41.1kJ?mol-1
�����Ѻϳɷ�Ӧ��
��iV��2 CH3OH(g) = CH3OCH3(g) + H2O(g) ��H4 = -24.5kJ?mol-1
�ش��������⣺
��1��Al2O3�Ǻϳ���ֱ���Ʊ������ѷ�Ӧ��������Ҫ�ɷ�֮һ����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ���������� ���Ի�ѧ����ʽ��ʾ����
��2�����������Ѻϳɷ�Ӧ��iV������COת���ʵ�Ӱ�� ��
��3����H2��COֱ���Ʊ������ѣ���һ����Ϊˮ���������Ȼ�ѧ����ʽΪ �����ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ�� ��
��4�����о����ڴ�������Cu��Zn��Al��O��Al2O3����ѹǿΪ5.0MPa�������£���H2��COֱ���Ʊ������ѣ��������ͼ��ʾ������COת�������¶����߶����͵�ԭ���� ��

��5��������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㣬�������ܶȵ��ڼ״�ֱ��ȼ�ϵ�أ�5.93kW?h?kg-1�����������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ ��һ�������ѷ��Ӿ����绯ѧ���������Բ��� �����ӵ��������õ�ص����������ѹΪ1.20V�������ܶ�E = ����ʽ���㡣�����ܶ�=����������/ȼ��������1 kW?h = 3.6��106J����
��1��Al2O3(������) + 2NaOH + 3H2O = 2NaAl(OH)4
NaAl(OH)4 + CO2 = Al(OH)3�� + NaHCO3��2Al(OH)3
 Al2O3+ 3H2O
Al2O3+ 3H2O��2�����ļ״����ٽ��״��ϳɷ�Ӧ��i��ƽ�����ƣ�COת�����������ɵ�H2O��ͨ��ˮú���任��Ӧ��iii�����IJ���CO��
��3��2CO(g) + 4H2(g) = CH3OCH3 + H2O(g) ��H = -204.7kJ?mol-1
�÷�Ӧ���������٣�ѹǿ����ʹƽ�����ƣ�CO��H2ת��������CH3OCH3�������ӡ�ѹǿ����ʹCO��H2Ũ�����ӣ���Ӧ��������
��4����Ӧ���ȣ��¶����ߣ�ƽ�����ơ�
��5��CH3OCH3 + 3H2O = 2CO2 + 12H+ + 12e- 12
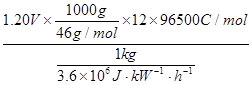 =8.39kW��h��kg-1
=8.39kW��h��kg-1��1�����������Ʊ��ϸߴ���Al2O3����Ҫ�������̣�������������������Һ�ܽ⣬Al2O3+ 2NaOH + 3H2O = 2NaAl(OH)4����Ԫ������Һ����NaAl(OH)4���ڣ�����Һ��ͨ�������̼��������ữ��NaAl(OH)4 + CO2 = Al(OH)3�� + NaHCO3�����˵õ�Al(OH)3�������������գ�2Al(OH)3
 Al2O3+ 3H2O���õ��ϸߴ���Al2O3��
Al2O3+ 3H2O���õ��ϸߴ���Al2O3����2�������ĸ���Ӧ����ʽ��֪�������Ѻϳɷ�Ӧ��iV�������ļ״����ٽ��״��ϳɷ�Ӧ��i��ƽ�����ƣ�COת�����������ɵ�H2O��ͨ��ˮú���任��Ӧ��iii�����IJ���CO��
��3��������Ŀ����������˹���ɣ���H2��COֱ���Ʊ������ѵ��Ȼ�ѧ����ʽΪ2CO(g) + 4H2(g) = CH3OCH3 + H2O(g) ��H = -204.7kJ?mol-1���÷�ӦΪ���������ٵķ�Ӧ������ѹǿʹƽ�����ƣ�CO��H2ת���ʾ�����CH3OCH3�������ӡ�����ѹǿʹCO��H2Ũ�����ӣ���Ӧ��������
��4����H2��COֱ���Ʊ������ѣ����ڸ÷�Ӧ�Ƿ��ȷ�Ӧ�����¶����ߣ�ƽ�������ƶ���COת�������¶����߶����͡�
��5�����ݷ�Ӧ���������ֱ��ȼ�ϵ�صĸ�����ӦΪCH3OCH3+3H2O-12e-=
2CO2+12H+�����ݷ���ʽ��֪:һ�������ѷ��Ӿ����绯ѧ���������Բ���12�����ӵ����������������ܶȵļ��㹫ʽ��E =
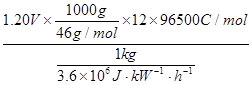 =8.39kW��h��kg-1��
=8.39kW��h��kg-1�������㶨λ����Ӧ���ʡ���ѧƽ�⡢�绯ѧ����ѧ����

��ϰ��ϵ�д�
�����Ŀ
 ��Ӧ����������ʾ��ͼ��
��Ӧ����������ʾ��ͼ��

 ����ʵ��ȼú��������Ļ��ա�ij�о�С����2Lij�ܱյ������������������������䣬��������������Բ��ƣ���ͨ��CO��SO2��10���Ӻ�������CO2�����ʵ���Ϊ0.9mol��
����ʵ��ȼú��������Ļ��ա�ij�о�С����2Lij�ܱյ������������������������䣬��������������Բ��ƣ���ͨ��CO��SO2��10���Ӻ�������CO2�����ʵ���Ϊ0.9mol��
 PCl3(g) + Cl2(g)��Ӧ����Ӧ������c(Cl2) ��ʱ��仯����������ͼ��ʾ������˵������ȷ����
PCl3(g) + Cl2(g)��Ӧ����Ӧ������c(Cl2) ��ʱ��仯����������ͼ��ʾ������˵������ȷ����
 H2(g) +CO2(g)ƽ�ⳣ��K���¶ȵı仯���±���
H2(g) +CO2(g)ƽ�ⳣ��K���¶ȵı仯���±���
 2C0 Cg)ƽ�ⳣ��K1��
2C0 Cg)ƽ�ⳣ��K1�� CO Cg) +H2(g)ƽ�ⳣ��K2
CO Cg) +H2(g)ƽ�ⳣ��K2

 Z(g)����60 s�ﵽƽ�⣬����0.3 mol Z������˵����ȷ����( )
Z(g)����60 s�ﵽƽ�⣬����0.3 mol Z������˵����ȷ����( ) Z��g���ﵽƽ�⣬����Ӧ������ʱ��ı仯��ͼ��ʾ��������������ȷ���ǣ� ��
Z��g���ﵽƽ�⣬����Ӧ������ʱ��ı仯��ͼ��ʾ��������������ȷ���ǣ� ��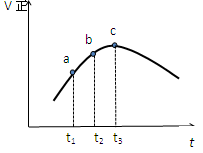
 2Z�� ��Ӧ�������еķ�Ӧ����(v)��ʱ��(t)�Ĺ�ϵ���ߣ�����������ȷ���� �� ��
2Z�� ��Ӧ�������еķ�Ӧ����(v)��ʱ��(t)�Ĺ�ϵ���ߣ�����������ȷ���� �� ��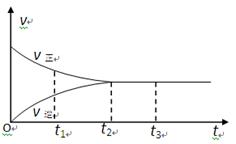
 CO(g)��3H2(g) ��H��0�����c(CH4)�淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������ж���ȷ����
CO(g)��3H2(g) ��H��0�����c(CH4)�淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������ж���ȷ����
 Br2(g)+H2(g)�������ֲ�ͬ�������½��У�Br2��H2��ʼ��Ũ��Ϊ0����Ӧ��HBr��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���
Br2(g)+H2(g)�������ֲ�ͬ�������½��У�Br2��H2��ʼ��Ũ��Ϊ0����Ӧ��HBr��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���