��Ŀ����
����Ŀ����������ƣ�Na2S2O3��5H2O�����������մ��ֳ�Ϊ������������������ˮ���������Ҵ������ȡ�������ֽ⡣ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ�����ͼ��

ʵ������������Ϊ��
�ٿ�����Һ©����ʹ�����������£��ʵ����ڷ�Һ�ĵ��٣�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ�����綯������������ˮԡ���ȣ��С�
��ֱ�������Ļ��Dz�����ʧ����������Һ��pH�ӽ�7ʱ��ֹͣͨ��SO2���塣
����
��1�������A������_______��
��2��Ϊ�˱�֤��������ƵIJ�����ʵ���в�������ҺpH <7���������ӷ���ʽ����ԭ��_________��
��3��д��������ƿB����ȡNa2S2O3����Ӧ���ܻ�ѧ��Ӧ����ʽ________��
��4����������������Һ�л�ýϸ߲���Na2S2O3��5H2O�IJ���Ϊ

Ϊ���ٲ�Ʒ����ʧ��������Ϊ���ȹ��ˣ�����������ԭ����______����������______���������dz��ˡ�ϴ�ӡ����
��5���ⶨ��Ʒ����
ȡ6.00g��Ʒ�����Ƴ�100mL��Һ��ȡ10.00mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.500mol/LI2�ı���Һ���еζ�����Ӧԭ��Ϊ2S2O32-+I2=S4O62-+2I-��������ݼ�¼���±���ʾ��
��� | 1 | 2 | 3 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.98 | 22.50 | 20.02 |
�ζ�ʱ���ﵽ�ζ��յ��������___________����Ʒ�Ĵ���Ϊ____________��
��6��Na2S2O3������������������Һ���ױ�Cl2������SO42-���÷�Ӧ�����ӷ���ʽΪ_________��
���𰸡� ������ƿ S2O32-+2H+=S��+SO2��+H2O 4SO2+2Na2S+Na2CO3=CO2+3Na2S2O3 Ϊ�˷�ֹ������©���д����������²��ʽ��� ����Ũ������ȴ�ᾧ ��Һ����ɫ����ɫ���Ұ���Ӳ���ɫ 82.67% S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+
����������1������A������������ƿ����2�����������Ϣ����������ƣ�Na2S2O3��5H2O����������Һ�в����ȶ����ڣ�������Ӧ�����ӷ���ʽΪS2O32-+ 2H+ == S��+ SO2��+H2O������ʵ���в�������ҺpH <7����3�����������Ϣ������ƿ�м�����Na2S��Na2CO3��Һ��ͨ����SO2���Ʊ�Na2S2O3��Na2S ����Ԫ����-2��ʧ��������Na2S2O3��SO2��SԪ����+4�۵õ�������Na2S2O3�����ݻ��ϼ�������ȡ�ԭ���غ���ƽ���ܷ�Ӧ�Ļ�ѧ����ʽΪ4SO2+2Na2S+Na2CO3 =CO2+3Na2S2O3����4�������¶��ܽ���������Ϊ���ٲ�Ʒ����ʧ��������Ϊ���ȹ��˵�ԭ����Ϊ�˷�ֹ������©���д����������²��ʽ��ͣ��������ǵõ����壬���ʵ�����������Ũ������ȴ�ᾧ����5��������������ɫ����ﵽ�ζ��յ����������Һ����ɫ����ɫ���Ұ���Ӳ���ɫ�����ݱ������ݿ�֪�ڶ���ʵ�����̫����ȥ�����ı�Һ���ƽ��ֵ��20.00mL�����ĵ�����ʵ�����0.0500mol/L��0.02L=0.001mol��������������Ƶ����ʵ�����0.002mol��������0.002mol��248g/mol��0.486g�����Ʒ�Ĵ���Ϊ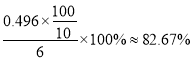 ����6��������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl����Ӧ�����ӷ���ʽΪS2O32��+ 4Cl2 + 5H2O��2SO42��+ 8Cl��+ 10H+ ��
����6��������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl����Ӧ�����ӷ���ʽΪS2O32��+ 4Cl2 + 5H2O��2SO42��+ 8Cl��+ 10H+ ��




