��Ŀ����
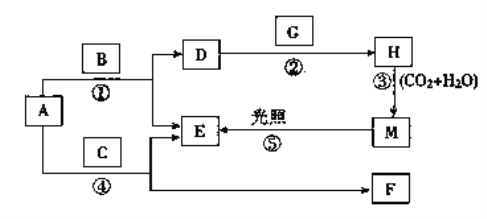
����Ŀ������A��B��C��D��E��F��G��H��M�������ʣ�����AΪ����ɫ��ĩ��BΪ�ճ������������ɫ��ζҺ�壬 EΪ��ɫ���嵥�ʣ�F��ˮ��Һ��ʯ��ˮ��Ͽɵ�D����Һ��GΪ����ɫ���嵥�ʣ�H��Ư��Һ����Ч�ɷ֣�����֮����ת����ϵ��ͼ��ʾ�����������ص������������ȥ��
��ش��������⣺
��1��д��G��H�Ļ�ѧʽ��G________��H________��D��������__________��
��2��д����Ӧ�ڵ����ӷ���ʽ��_________________________________________��
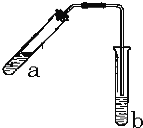
��3��������ͼװ�ý���ʵ�飬֤��������ǿ����KMnO4>Cl2>Br2��
��ѡ�Լ���KBr��Һ��KMnO4��Ũ���ᡣ

��֪��2KMnO4+16HCl(Ũ)=2KCl+ 2MnCl2+5Cl2��+8H2O
��ش��������⣺
װ��a ��������____________________��d��ʢ�ŵ��Լ���____________��
���𰸡� Cl2 NaClO �ռ� Cl2+2OH��Cl+ ClO+ H2O ��Һ©�� KBr��Һ
��������AΪ����ɫ��ĩ��A�ǹ������ơ�BΪ�ճ������������ɫ��ζҺ�壬B��ˮ������������ˮ��Ӧ�����������ƺ�������EΪ��ɫ���嵥�ʣ�E��������D���������ơ�GΪ����ɫ���嵥�ʣ�H��Ư��Һ����Ч�ɷ֣�G��������H�Ǵ������ơ�����������Һ���ն�����̼����M�Ǵ����ᣬ���������ֽ������������Ȼ��⡣F��ˮ��Һ��ʯ��ˮ��Ͽɵ�D����Һ��F��̼���ƣ����C�Ƕ�����̼��
��1���������Ϸ�����֪G��H�Ļ�ѧʽ�ֱ���Cl2��NaClO���������Ƶ��������ռ�����2����Ӧ�ڵ����ӷ���ʽΪCl2+2OH��Cl+ClO+H2O����3��װ��a�������Ƿ�Һ©�������Ը��������Һ����Ũ���������������������廯������Ϊ�����壬��˿�����֤������ǿ��������d��ʢ�ŵ��Լ���KBr��Һ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�