ΧβΡΩΡΎ»ί
“―÷ΣΘΚP4(g)ΘΪ6Cl2(g)=4PCl3(g)ΓΓΠΛHΘΫa kJΓΛmolΘ≠1Θ§P4(g)ΘΪ10Cl2(g)=4PCl5(g)ΓΓΠΛHΘΫb kJΓΛmolΘ≠1Θ§≤Δ÷Σ–Έ≥…Μρ≤πΩΣ1 molΜ·―ßΦϋΖ≈≥ωΜρΈϋ ’ΒΡΡήΝΩ≥ΤΈΣΗΟΜ·―ßΦϋΒΡΦϋΡήΓΘP4ΨΏ”–’ΐΥΡΟφΧεΫαΙΙΘ§PCl5÷–PΘ≠ClΦϋΒΡΦϋΡήΈΣc kJΓΛmolΘ≠1Θ§PCl3÷–PΘ≠ClΦϋΒΡΦϋΡήΈΣ1.2c kJΓΛmolΘ≠1ΓΘœ¬Ν––π ω’ΐ»ΖΒΡ «Θ®?? Θ©
AΘ°PΘ≠PΦϋΒΡΦϋΡή¥σ”ΎPΘ≠ClΦϋΒΡΦϋΡή
BΘ°Ω…«σCl2(g)ΘΪPCl3(g)=PCl5(s)ΒΡΖ¥”Π»»ΠΛH
CΘ°ClΘ≠ClΦϋΒΡΦϋΡήΈΣ(bΘ≠aΘΪ5.6c)/4 kJΓΛmolΘ≠1
DΘ°PΘ≠PΦϋΒΡΦϋΡήΈΣ(5aΘ≠3bΘΪ12c)/8 kJΓΛmolΘ≠1
C
ΓΨΫβΈωΓΩClΒΡΖ«Ϋπ τ–‘¥σ”ΎPΒΡΖ«Ϋπ τ–‘Θ§Ι PΓΣClΦϋΒΡΦϋΡή¥σ”ΎPΓΣPΦϋΒΡΦϋΡήΘ§Aœν¥μΈσΘΜ≤Μ÷ΣΒάPCl5(g)ΓζPCl5(s)ΒΡΖ¥”Π»»Θ§ΈόΖ®«σ≥ωBœν÷–Ζ¥”ΠΒΡΖ¥”Π»»Θ§Bœν¥μΈσΘΜΗυΨίΗ«ΥΙΕ®¬…Θ§œϊ»ΞP4Θ§ΒΟΒΫClΓΣClΦϋΒΡΦϋΡήΈΣ(bΘ≠aΘΪ5.6c)/4 kJΓΛmolΘ≠1Θ§Cœν’ΐ»ΖΘΜΗυΨίΗ«ΥΙΕ®¬…Θ§œϊ»ΞCl2Θ§ΒΟΒΫPΓΣPΦϋΒΡΦϋΡήΈΣ(5aΘ≠3bΘΪ12c)/12 kJΓΛmolΘ≠1Θ§Dœν¥μΈσΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΔώ.œ¬Ν–”–ΙΊ Β―ι≤ΌΉςΜρΫαΙϊΒΡΥΒΖ®÷–’ΐ»ΖΒΡ « Θ®ΧνΉ÷ΡΗΘ©
| AΘ°ΒΈΕ® ±Θ§―έΨΠ”Π Φ÷’ΉΔ ”ΒΈΕ®ΙήΡΎ“ΚΟφΒΡ±δΜ· |
| BΘ°”ΟΦν ΫΒΈΕ®ΙήΝΩ»Γ0.10 molΓΛLΘ≠1ΒΡKMnO4»ή“Κ15.10 mL |
| CΘ°ΥαΦν÷–ΚΆΒΈΕ®÷°«ΑΘ§ΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΨΜΦ¥Ω…Θ§≤ΜΡή”Ο¥ΐ≤β“Κ»σœ¥ |
| DΘ°”ΟpH ‘÷Ϋ≤βΝΩΡ≥»ή“ΚΒΡpH ±“Σœ»ΫΪ ‘÷Ϋ»σ Σ |
FΘ°”ΟΙψΖΚpH ‘÷Ϋ≤βΝΩH2SO4»ή“ΚΒΡpH ±Θ§≤βΒΟpH=3.2
GΘ°≤βΕ®ΥαΦνΒΈΕ®«ζœΏΘΚΩΣ Φ ±≤β ‘ΚΆΦ«¬ΦΒΡΦδΗτΩ……‘–Γ–©Θ§ΒΈΕ®÷Ν÷’ΒψΗΫΫϋ‘ρ“Σ¥σ–©
Δρ.Θ®1Θ©”…«βΤχΚΆ―θΤχΖ¥”Π…ζ≥…1 molΥ°’τΤχΖ≈»»241.8kJΘ§–¥≥ωΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ ΓΘ“―÷ΣH2O(l) ΘΫ H2O(g) ΠΛH ΘΫΘΪ44 kJΓΛmolΘ≠1‘ρ±ξΉΦΉ¥Ωωœ¬33.6 L H2…ζ≥…“ΚΧ§Υ° ±Ζ≈≥ωΒΡ»»ΝΩ « kJΓΘ
Θ®2Θ©Μ·―ßΖ¥”ΠΩ… ”ΈΣΨ…ΦϋΕœΝ―ΚΆ–¬Φϋ–Έ≥…ΒΡΙΐ≥ΧΓΘΜ·―ßΦϋΒΡΦϋΡή «–Έ≥…Θ®Μρ≤πΩΣΘ©1molΜ·―ßΦϋ ± ΆΖ≈Θ®ΜρΈϋ ’Θ©ΒΡΡήΝΩΓΘ“―÷ΣΑΉΝΉP4ΚΆP4O6ΒΡΖ÷Ή”ΫαΙΙ»γœ¬ΆΦΥυ ΨΘΚ
œ÷ΧαΙ©“‘œ¬Μ·―ßΦϋΒΡΦϋΡήΘΚP-P 198 kJΓΛmolΘ≠1 P-O 360 kJΓΛmolΘ≠1,―θΤχΖ÷Ή”ΡΎ―θ‘≠Ή”ΦδΒΡΘ®O=OΘ©ΦϋΡήΈΣ498 kJΓΛmolΘ≠1ΓΘ‘ρP4+3O2 = P4O6ΒΡΖ¥”Π»»ΠΛHΈΣ ΓΘ











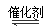 CH3OCH3ΘΪH2O
CH3OCH3ΘΪH2O CH3OCH3(g)ΘΪCO2(g) ΓςHΘΫΘ≠247kJ/mol
CH3OCH3(g)ΘΪCO2(g) ΓςHΘΫΘ≠247kJ/mol

