��Ŀ����
�����ѣ�CH3OCH3����һ����Ҫ�ľ�ϸ������Ʒ������Ϊ�Ƕ�ʮһ��������DZ����ȼ��[ ��֪��CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O��1��? ��H����1455kJ/mol ]��ͬʱ��Ҳ������Ϊ�������������ȴ�������ҵ���Ʊ������ѵ���Ҫ���������������Σ�
���״�Һ����Ũ���������»�״������ڴ�������ֱ����ˮ�ƶ����ѣ� 2CH3OH  CH3OCH3��H2O
CH3OCH3��H2O
���ϳ���CO��H2ֱ�Ӻϳɶ����ѣ� 3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)?? ��H����247kJ/mol
CH3OCH3(g)��CO2(g)?? ��H����247kJ/mol
����Ȼ����ˮ������Ӧ�Ʊ������ѡ���CH4��H2OΪԭ���Ʊ������Ѻͼ״���ҵ�������£�

��1��д��CO(g)��H2(g)��O2(g)��Ӧ����CO2(g)��H2O��1�����Ȼ�ѧ����ʽ���������һλС����????????????????????????????????????????????????
��2���ڷ�Ӧ��2�У�һ�������·�����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��?????
CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��?????
A�����¸�ѹ?? B���Ӵ���??? C������COŨ��?? D�������������
��3���ڷ�Ӧ��3�У���һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��3H2(g)��CO2(g)  CH3OH(g)��H2O (g)? ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ����??? ������ţ�
CH3OH(g)��H2O (g)? ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ����??? ������ţ�

A��P3��P2?? T3��T2?????? B��P2��P4?? T4��T2
C��P1��P3 ? T1��T3?????? D��P1��P4?? T2��T3
��4����Ӧ��1�з�����Ӧ��CH4(g)��H2O(g) CO(g)��3H2(g)? ��H��0д��ƽ�ⳣ���ı���ʽ��??????????????????????????
CO(g)��3H2(g)? ��H��0д��ƽ�ⳣ���ı���ʽ��??????????????????????????
����¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ��????????????? ���������������������������С����

��5����ͼΪ��ɫ��Դ��������ȼ�ϵ�����Ĺ���ԭ��ʾ��ͼ��a�缫�ķ�ӦʽΪ��________________

��6�������ж�����ȷ����_______
A�����ձ�a�м�������K3[Fe(CN)6]��Һ������ɫ��������
B���ձ�b�з�����ӦΪ2Zn-4e�� ��2Zn2+
C�����Ӵ�Zn������������Fe���������Żص�Zn��
D���ձ�a�з�����ӦO2 + 4H+ + 4e�� �� 2H2O����ҺpH����
��1��CO(g)��H2(g)��O2(g) CO2(g)��H2O(l)? ��H����567.3kJ/mol
CO2(g)��H2O(l)? ��H����567.3kJ/mol
��2��AD? ��3��C D?? ��4��k�� ����С
����С
��5��CH3OCH3��12e��+16OH����2CO32��+11 H2O��6��B
��������
�����������1����CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O��1�� ��H����1455kJ/mol ��3H2(g)��3CO(g)  CH3OCH3(g)��CO2(g)?? ��H����247kJ/mol����������ɵ���CO(g)��H2(g)��O2(g)
CH3OCH3(g)��CO2(g)?? ��H����247kJ/mol����������ɵ���CO(g)��H2(g)��O2(g) CO2(g)��H2O(l)? ��H����567.3kJ/mol����2��������Ӧ3H2(g)��3CO(g)
CO2(g)��H2O(l)? ��H����567.3kJ/mol����2��������Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g) ��H����247kJ/mol������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����Դﵽƽ���Ҫ���CO��ת���ʣ�����ƽ�������ƶ���Ӧ�õ��¸�ѹ�����������ѡ�����Ӵ���ֻ�����̴ﵽƽ������Ҫ��ʱ�䣬��ƽ�ⲻ�����ƶ�����??? ����COŨ�ȣ�ƽ�������ƶ���ת�������ӣ���������ԶԶ����ƽ��ת��������������CO��ת���ʷ������͡�ѡ��ΪAD����3����Ӧ3H2(g)��CO2(g)
CH3OCH3(g)��CO2(g) ��H����247kJ/mol������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����Դﵽƽ���Ҫ���CO��ת���ʣ�����ƽ�������ƶ���Ӧ�õ��¸�ѹ�����������ѡ�����Ӵ���ֻ�����̴ﵽƽ������Ҫ��ʱ�䣬��ƽ�ⲻ�����ƶ�����??? ����COŨ�ȣ�ƽ�������ƶ���ת�������ӣ���������ԶԶ����ƽ��ת��������������CO��ת���ʷ������͡�ѡ��ΪAD����3����Ӧ3H2(g)��CO2(g)  CH3OH(g)��H2O (g)? ��H��0�ﵽƽ���������ѹǿ���¶ȣ�ƽ�������ƶ���CH3OH�ĺ������ӣ�������ѹǿ�������¶ȣ�ƽ�������ƶ���CH3OH�ĺ������١���A.P2>P3��T2>T3.����B��P2��P4?? T2��T4.����C��P1��P3 ���� T1��T3 ��ȷ��D��P1��P4 ��T2��T3����ȷ����4����CH4(g)��H2O(g)
CH3OH(g)��H2O (g)? ��H��0�ﵽƽ���������ѹǿ���¶ȣ�ƽ�������ƶ���CH3OH�ĺ������ӣ�������ѹǿ�������¶ȣ�ƽ�������ƶ���CH3OH�ĺ������١���A.P2>P3��T2>T3.����B��P2��P4?? T2��T4.����C��P1��P3 ���� T1��T3 ��ȷ��D��P1��P4 ��T2��T3����ȷ����4����CH4(g)��H2O(g) CO(g)��3H2(g)? ��H��0�Ļ�ѧƽ�ⳣ���ı���ʽΪk��
CO(g)��3H2(g)? ��H��0�Ļ�ѧƽ�ⳣ���ı���ʽΪk�� ����¶Ƚ��ͣ�����ƽ���ƶ�ԭ������ѧƽ������ȷ�Ӧ����(�����淴Ӧ����)�ƶ������Ը÷�Ӧ��ƽ�ⳣ����С����5����ȼ�ϵ����������ȼ�ϵ�����У�ͨ��ȼ�ϵĵ缫a�缫Ϊ�������缫�ķ�ӦʽΪCH3OCH3��12e��+16OH����2CO32��+11 H2O����6��A.���ڻ��Zn>Fe,������Znʧȥ���ӣ���������Fe����������ΪFeδ����������Ӧ���������ձ�a�м�������K3[Fe(CN)6]��Һ����������ɫ�������ɡ�A����B��ȷ��C. ���Ӵ�Zn������������Fe��������Һ�������ӴӸ����������ƶ��������Ӵ������ฺ���ƶ����Ӷ��γɱպϻ�·������.D��NaCl��Һ�����ԣ��������ձ�a�з�����ӦO2 + 2H2O + 4e�� ��4OH-����ҺpH���ߡ�����
����¶Ƚ��ͣ�����ƽ���ƶ�ԭ������ѧƽ������ȷ�Ӧ����(�����淴Ӧ����)�ƶ������Ը÷�Ӧ��ƽ�ⳣ����С����5����ȼ�ϵ����������ȼ�ϵ�����У�ͨ��ȼ�ϵĵ缫a�缫Ϊ�������缫�ķ�ӦʽΪCH3OCH3��12e��+16OH����2CO32��+11 H2O����6��A.���ڻ��Zn>Fe,������Znʧȥ���ӣ���������Fe����������ΪFeδ����������Ӧ���������ձ�a�м�������K3[Fe(CN)6]��Һ����������ɫ�������ɡ�A����B��ȷ��C. ���Ӵ�Zn������������Fe��������Һ�������ӴӸ����������ƶ��������Ӵ������ฺ���ƶ����Ӷ��γɱպϻ�·������.D��NaCl��Һ�����ԣ��������ձ�a�з�����ӦO2 + 2H2O + 4e�� ��4OH-����ҺpH���ߡ�����
���㣺�����Ȼ�ѧ����ʽ����д����������Ի�ѧƽ���Ӱ�졢��ѧƽ�ⳣ���ĺ��弰Ӧ�á�ԭ��ع���ԭ���������жϵ�֪ʶ��

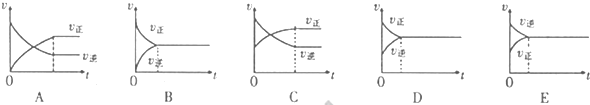

 CO��g��+3H2��g��������I����CO��g��+H2O��g��
CO��g��+3H2��g��������I����CO��g��+H2O��g�� ��100��������Ӧ��������ԭ��������Ϊ ��
��100��������Ӧ��������ԭ��������Ϊ ��
 ��100��������Ӧ��������ԭ��������Ϊ ��
��100��������Ӧ��������ԭ��������Ϊ ��
 ��H2�ϳɶ����ѵĻ�ѧ����ʽΪ .
��H2�ϳɶ����ѵĻ�ѧ����ʽΪ . CO��g��+3H2��g��������I����CO��g��+H2O��g��
CO��g��+3H2��g��������I����CO��g��+H2O��g�� ��100��������Ӧ��������ԭ��������Ϊ ��
��100��������Ӧ��������ԭ��������Ϊ ��