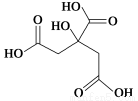
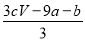ΧβΡΩΡΎ»ί
¥σΤχ÷–ΒΡ≤ΩΖ÷Ββ‘¥”ΎO3Ε‘ΚΘΥ°÷–IΘ≠ΒΡ―θΜ·Θ§ΫΪO3≥÷–χΆ®»κNaI»ή“Κ÷–Ϋχ––ΡΘΡβ―–ΨΩΓΘ
(1)O3ΫΪIΘ≠―θΜ·≥…I2ΒΡΙΐ≥Χ”…3≤ΫΖ¥”ΠΉι≥…ΘΚ
ΔΌIΘ≠(aq)ΘΪO3(g)=IOΘ≠(aq)ΘΪO2(g)ΠΛH1
ΔΎIOΘ≠(aq)ΘΪHΘΪ(aq)  HOI(aq)ΠΛH2
HOI(aq)ΠΛH2
ΔέHOI(aq)ΘΪIΘ≠(aq)ΘΪHΘΪ(aq)  I2(aq)ΘΪH2O(l)ΠΛH3
I2(aq)ΘΪH2O(l)ΠΛH3
ΉήΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________Θ§ΤδΖ¥”Π»»ΠΛHΘΫ__________ΓΘ
(2)‘Ύ»ή“Κ÷–¥φ‘ΎΜ·―ßΤΫΚβΘΚI2(aq)ΘΪIΘ≠(aq)  I3ΓΣ(aq)Θ§
I3ΓΣ(aq)Θ§
ΤδΤΫΚβ≥Θ ΐ±μ¥ο ΫΈΣ______________ΓΘ
(3)ΈΣΧΫΨΩFe2ΘΪΕ‘O3―θΜ·IΘ≠Ζ¥”ΠΒΡ”Αœλ(Ζ¥”ΠΧεœΒ»γΆΦ1)Θ§Ρ≥―–ΨΩ–ΓΉι≤βΕ®ΝΫΉι Β―ι÷–I3ΓΣ≈®Ε»ΚΆΧεœΒpHΘ§ΫαΙϊΦϊΆΦ2ΚΆœ¬±μΓΘ
 ΓΔ
ΓΔ
±ύΚ≈ | Ζ¥”ΠΈο | Ζ¥”Π«ΑpH | Ζ¥”ΠΚσpH |
ΒΎ1Ήι | O3ΘΪIΘ≠ | 5.2 | 11.0 |
ΒΎ2Ήι | O3ΘΪIΘ≠ΘΪFe2ΘΪ | 5.2 | 4.1 |
ΔΌΒΎ1Ήι Β―ι÷–Θ§ΒΦ÷¬Ζ¥”ΠΚσpH…ΐΗΏΒΡ‘≠“ρ «______________________________ΓΘ
ΔΎΆΦ1÷–ΒΡAΈΣ__________Θ§”…Fe3ΘΪ…ζ≥…AΒΡΙΐ≥ΧΡήœ‘÷χΧαΗΏIΘ≠ΒΡΉΣΜ·¬ Θ§‘≠“ρ «____________________________________________________________ΓΘ
ΔέΒΎ2Ήι Β―ιΫχ––18 sΚσΘ§I3ΓΣ≈®Ε»œ¬ΫΒΘ§ΒΦ÷¬œ¬ΫΒΒΡ÷±Ϋ”‘≠“ρ”–(ΥΪ―Γ)________(ΧνΉ÷ΡΗ–ρΚ≈)ΓΘ
AΘ°c(HΘΪ)Φθ–Γ BΘ°c(IΘ≠)Φθ–Γ CΘ°I2(g)≤ΜΕœ…ζ≥… DΘ°c(Fe3ΘΪ)‘ωΦ”
(4)ΨίΆΦ2Θ§ΦΤΥψ3ΓΪ18 sΡΎΒΎ2Ήι Β―ι÷–…ζ≥…I3ΓΣΒΡΤΫΨυΖ¥”ΠΥΌ¬ (–¥≥ωΦΤΥψΙΐ≥ΧΘ§ΫαΙϊ±ΘΝτΝΫΈΜ”––ß ΐΉ÷)ΓΘ
(1)2IΘ≠(aq)ΘΪO3(g)ΘΪ2HΘΪ(aq)  I2(aq)ΘΪO2(g)ΘΪH2O(l)ΓΓΠΛH1ΘΪΠΛH2ΘΪΠΛH3
I2(aq)ΘΪO2(g)ΘΪH2O(l)ΓΓΠΛH1ΘΪΠΛH2ΘΪΠΛH3
(2)KΘΫ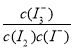
(3)ΔΌ”…”Ύ2IΘ≠ΘΪO3ΘΪ2HΘΪ=I2ΘΪO2ΘΪH2OΘ§Φ¥œϊΚΡHΘΪ”÷…ζ≥…Υ°Θ§ΒΦ÷¬»ή“ΚΒΡΥα–‘Φθ»θΘ§pH…ΐΗΏ
ΔΎFe2ΘΪΓΓ”…”Ύ2Fe3ΘΪΘΪ2IΘ≠=I2ΘΪ2Fe2ΘΪΘ§ Ιc(I2)‘ω¥σΘ§¥Ό ΙI2(aq)ΘΪIΘ≠(aq)  I3ΓΣ(aq)Θ§ΤΫΚβ”““ΤΘ§œϊΚΡΒΡc(IΘ≠)‘ωΕύ
I3ΓΣ(aq)Θ§ΤΫΚβ”““ΤΘ§œϊΚΡΒΡc(IΘ≠)‘ωΕύ
ΔέBD
(4)v(I3ΓΣ)ΘΫ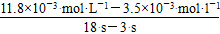 Γ÷5.5ΓΝ10Θ≠4 molΓΛLΘ≠1ΓΛsΘ≠1
Γ÷5.5ΓΝ10Θ≠4 molΓΛLΘ≠1ΓΛsΘ≠1
ΓΨΫβΈωΓΩάϊ”ΟΗ«ΥΙΕ®¬…ΓΔΜ·―ßΖ¥”ΠΥΌ¬ ΓΔΜ·―ßΤΫΚβΒΡ”Αœλ“ρΥΊΒ»Ζ÷ΈωΦΑΦΤΥψΘ§÷π≤ΫΫβΨωΈ ΧβΓΘ(1)ΗυΨίΗ«ΥΙΕ®¬…Θ§”…ΔΌΘΪΔΎΘΪΔέΩ…ΒΟΉήΖ¥”ΠΈΣ2IΘ≠(aq)ΘΪO3(g)ΘΪ2HΘΪ(aq)=I2(aq)ΘΪO2(g)ΘΪH2O(l)Θ§‘ρΠΛHΘΫΠΛH1ΘΪΠΛH2ΘΪΠΛH3ΓΘ
(2)ΥυΗχΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ±μ¥ο ΫΈΣKΘΫ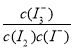 ΓΘ(3)ΔΌΒΎ1Ήι Β―ι÷– Θ§pH…ΐΗΏ «“ρΈΣΖ¥”ΠœϊΚΡΝΥHΘΪΓΘΔΎΆΦ1÷–ΒΡAΈΣFe2ΘΪΘ§”…Fe3ΘΪ…ζ≥…Fe2ΘΪΒΡΙΐ≥Χ÷–Θ§IΘ≠±Μ―θΜ·ΈΣI2Θ§“ρ¥ΥIΘ≠ΒΡΉΣΜ·¬ œ‘÷χΧαΗΏΓΘΔέΒΦ÷¬I3ΓΣ≈®Ε»œ¬ΫΒΒΡ‘≠“ρ «c(Fe3ΘΪ)‘ωΦ”Θ§ Ιc(IΘ≠)Φθ–ΓΘ§ΤΫΚβI2(aq)ΘΪIΘ≠(aq)
ΓΘ(3)ΔΌΒΎ1Ήι Β―ι÷– Θ§pH…ΐΗΏ «“ρΈΣΖ¥”ΠœϊΚΡΝΥHΘΪΓΘΔΎΆΦ1÷–ΒΡAΈΣFe2ΘΪΘ§”…Fe3ΘΪ…ζ≥…Fe2ΘΪΒΡΙΐ≥Χ÷–Θ§IΘ≠±Μ―θΜ·ΈΣI2Θ§“ρ¥ΥIΘ≠ΒΡΉΣΜ·¬ œ‘÷χΧαΗΏΓΘΔέΒΦ÷¬I3ΓΣ≈®Ε»œ¬ΫΒΒΡ‘≠“ρ «c(Fe3ΘΪ)‘ωΦ”Θ§ Ιc(IΘ≠)Φθ–ΓΘ§ΤΫΚβI2(aq)ΘΪIΘ≠(aq)  I3ΓΣ(aq)Ρφœρ“ΤΕ·ΓΘ
I3ΓΣ(aq)Ρφœρ“ΤΕ·ΓΘ
(4)v(I3ΓΣ)ΘΫ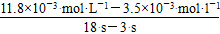 Γ÷5.5ΓΝ10Θ≠4 molΓΛLΘ≠1ΓΛsΘ≠1ΓΘ
Γ÷5.5ΓΝ10Θ≠4 molΓΛLΘ≠1ΓΛsΘ≠1ΓΘ