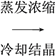��Ŀ����
����Ŀ����������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⡣(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�д������ڵ���������________��Y�����Ũ���ᷴӦ����Һ�к��е��εĻ�ѧʽΪ________��
(2)ij��Һ����Mg2����Fe2����Al3����Cu2�������ӣ������м��������NaOH��Һ���ˣ��������������գ��������պ�Ĺ���Ͷ�������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2�������� B��Fe2��������
C��Al3�������� D��Cu2��
(3)����������Ҫ�Ĺ�ҵ���ϣ��÷���м�Ʊ������������£�
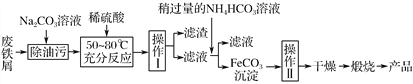
�ش��������⣺
�ٲ�������������________����������������________���������ķ���Ϊ________��
����д������FeCO3���������ӷ���ʽ��___________________________________��
���𰸡�(1)Fe2����Fe3����H��Fe(NO3)3
(2)BC
(3)������ ϴ�� ��©���м�����������ˮ����û������������ˮ��Ȼ���£��ظ�2��3��
��Fe2����2HCO3-=FeCO3����CO2����H2O
��������(1)�����������о��д��Ե���Fe3O4��Fe3O4���ڹ������������Һ�д��ڵ���������Fe2����Fe3����H����Fe3O4���ڹ�����Ũ�����FeԪ�ػᱻ����ΪFe3�������Է�Ӧ�����Һ�к��е�����Fe(NO3)3��(2)�����Һ�м��������NaOH��Һ��Mg2����Mg(OH)2��Fe2����Fe(OH)3��Al3����[AlCO4]����Cu2����Cu(OH)2�����˺������к���Mg(OH)2��Fe(OH)3��Cu(OH)2���������պ�Mg(OH)2��MgO��Fe(OH)3��Fe2O3��Cu(OH)2��CuO����MgO��Fe2O3��CuOͶ��������������Mg2����Fe3����Cu2������Ӧѡ��B��C��(3)��������ͼ��֪�����������õ��������ʸò����ǹ��ˣ����������ڵõ�FeCO3��������еģ��ʸò�����ϴ�ӣ�ϴ�Ӿ����������Ϊ����©���м�����������ˮ����û������������ˮ��Ȼ���£��ظ�2��3�Σ���������ͼ�з��������Թ�����NH4HCO3��Һ����FeCO3��������Ӧ��CO2���ɣ��������ֽⷴӦ�����ӷ���ʽΪ��Fe2����2HCO3-=FeCO3����CO2����H2O��

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
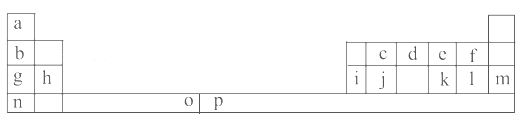
�Իش��������⣺
��1��Ԫ��pΪ26��Ԫ�أ���д����ԭ�ӵ����������ӵĵ����Ų�ʽ��______________��
��2��e��a��Ӧ�IJ���ķ���������ԭ�ӵ��ӻ���ʽΪ__________���÷�����__________(����ԡ��Ǽ��ԡ�)���ӡ�
��3����д��f���⻯����ˮ������������ı���ʽ������д���ּ��ɣ�_________________________��
��4��o��p��Ԫ�صIJ��ֵ��������������±���
Ԫ�� | o | p | |
������ /kJ��mol��1 | I1 | 717 | 759 |
I2 | 1 509 | 1 561 | |
I3 | 3 248 | 2 957 | |
�Ƚ���Ԫ�ص�I2��I3��֪����̬o2����ʧȥһ�����ӱ���̬p2����ʧȥһ�������ѡ��Դˣ���Ľ�����___________________________________��
(5)i���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
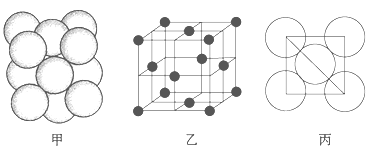
���ʾ�����iԭ�ӵ���λ��Ϊ________��һ��������iԭ�ӵ���ĿΪ________��