��Ŀ����
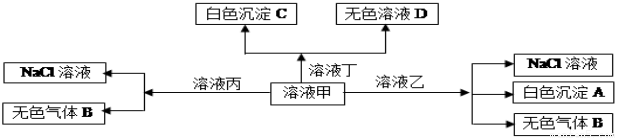
ij��Һ�к��������������е�������:K+��Mg2+��Fe3+��Fe2+��CO32-��NO3-��SO42-��I-��SiO32-��Cl-�������ʵ���Ũ����ͬ��ijͬѧ��̽������Һ�����,����������ʵ��:
��.�ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ���,�۲쵽dz��ɫ���棻
��.��ȡԭ��Һ����������������ɫ�������ɣ���������������ɺ���ɫ����ʱ��Һ��ɫ������������ɣ�
��.ȡ��Ӧ�����Һ��������֧�Թ��У���һ֧�Թ��м���BaCl2��Һ���а�ɫ��������,�ٵμ�KSCN��Һ�ϲ���Һ���;�ڶ�֧�Թ��м���CCl4��������ú���Һ�ֲ㣬�²�����Ϻ�ɫ������˵����ȷ���ǣ� ��
A��ԭ��Һ�п϶�����Mg2+��SiO32-
B�����������ɫ������ܺ���CO2,ԭ��Һ�п��ܺ���CO32-
C��ԭ��Һ��K+��Fe2+��NO3-��I-��SO42-�����������
D��ԭ��Һ��һ������Mg2+��Cl-
��ϰ��ϵ�д�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
�����Ŀ


 ��Ӧ�õ�4molCH3COOH��1molB.����˵���������
��Ӧ�õ�4molCH3COOH��1molB.����˵��������� CH3CH2OH(g)+H2O(g) ��H1
CH3CH2OH(g)+H2O(g) ��H1 ��Դ�״�����Ӧ��CO(g)+2H2(g)
��Դ�״�����Ӧ��CO(g)+2H2(g)