��Ŀ����
��13�֣���1���������Ż�_________����ܡ����ܡ�����ˮ���ԭ����________________________________________________________���û�ѧ��Ӧ�����Խ��ͣ���______����ܡ����ܡ�������ĭ��������ԭ����____________________________���û�ѧ��Ӧ�����Խ��ͣ���
��2������H1N1���в�����ȫ��㷺�����������ཡ������ᾭ�ô����˾�ĸ���Ӱ�졣�ҹ���ȡ����Ӧ�Դ�ʩ��ʹ�����õ�����Ч���ƣ��Ӻܴ�̶��ϼ�������ʧ�����������Һ������û������������Һ�ǽ�����ͨ��NaOH��Һ�У����������ӷ�Ӧ����ʽΪ_____________________________________��
������Һϡ�ͺ������ڿ����У�����������Ư���Ե����ʣ���д���˹��̵����ӷ�Ӧ����ʽ_____________________________________����������Ư��������Ϊ����ǿ�����ԣ��������ܲ��ȶ�����д�����ֽ�Ļ�ѧ����ʽ_____________________________________��
��2������H1N1���в�����ȫ��㷺�����������ཡ������ᾭ�ô����˾�ĸ���Ӱ�졣�ҹ���ȡ����Ӧ�Դ�ʩ��ʹ�����õ�����Ч���ƣ��Ӻܴ�̶��ϼ�������ʧ�����������Һ������û������������Һ�ǽ�����ͨ��NaOH��Һ�У����������ӷ�Ӧ����ʽΪ_____________________________________��
������Һϡ�ͺ������ڿ����У�����������Ư���Ե����ʣ���д���˹��̵����ӷ�Ӧ����ʽ_____________________________________����������Ư��������Ϊ����ǿ�����ԣ��������ܲ��ȶ�����д�����ֽ�Ļ�ѧ����ʽ_____________________________________��
(1)����1�֣�2Na+2H2O=2NaO H+H2��1�� 2Na2O2+2H2O=4NaOH+O2��1�֣�����1�֣�2Na2O2+2CO2=2Na2CO3+O2 1
H+H2��1�� 2Na2O2+2H2O=4NaOH+O2��1�֣�����1�֣�2Na2O2+2CO2=2Na2CO3+O2 1 �֡�
�֡�
��2��Cu+4HNO3=Cu(NO3)2+2NO2��+2H2O2�֣�3NO2+H2O=2HNO3+NO1�֣�
��3����Cl2+2OH-=Cl-+ClO-+H2O2�֢�CO2+H2O+2ClO-=2HClO+CO32-2�֣�2HClO=2HCl+O21��
 H+H2��1�� 2Na2O2+2H2O=4NaOH+O2��1�֣�����1�֣�2Na2O2+2CO2=2Na2CO3+O2 1
H+H2��1�� 2Na2O2+2H2O=4NaOH+O2��1�֣�����1�֣�2Na2O2+2CO2=2Na2CO3+O2 1 �֡�
�֡���2��Cu+4HNO3=Cu(NO3)2+2NO2��+2H2O2�֣�3NO2+H2O=2HNO3+NO1�֣�
��3����Cl2+2OH-=Cl-+ClO-+H2O2�֢�CO2+H2O+2ClO-=2HClO+CO32-2�֣�2HClO=2HCl+O21��
��

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ
 �����ͨ���������õ��Ĺ�ͬ����Ҫԭ���� �� ��
�����ͨ���������õ��Ĺ�ͬ����Ҫԭ���� �� �� �͡��л�������һ�� �� ���ϣ�ѡ������Ρ�����ά�������ϡ������������ϵĽṹ�ɱ�ʾΪ
�͡��л�������һ�� �� ���ϣ�ѡ������Ρ�����ά�������ϡ������������ϵĽṹ�ɱ�ʾΪ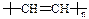 �����䵥��Ľṹ��ʽΪ �� ��
�����䵥��Ľṹ��ʽΪ �� ��