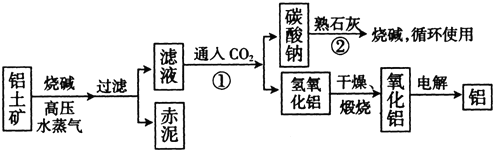
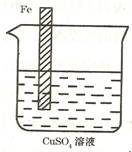
��Ŀ����
�������ڵؿ��еĺ������������裬���ڵ���λ�͵���λ���ִ������벻�����ǣ��������������ʼ��仯��������ʺ���;���ش��������⣮
��1���������ȷ�Ӧԭ�����Ӹֹ죬�û�ѧ����ʽ��ʾ ��
��2�����������Ǽ���������ˮ�İ�ɫ��״�����������ǿ���ǿ����Һ���������ӷ���ʽ��ʾ ��
��3������[KAl��SO4��2?12H2O]�����ھ�ˮ����������ˮ��Һ�е�����ɫʯ����Һ����Һ�� ɫ��������Ϊ�������ӷ���ʽ��ʾ�� ��
��4�������ȵ���˿����ˮ���û�ѧ����ʽ��ʾ�� ��
��1���������ȷ�Ӧԭ�����Ӹֹ죬�û�ѧ����ʽ��ʾ
��2�����������Ǽ���������ˮ�İ�ɫ��״�����������ǿ���ǿ����Һ���������ӷ���ʽ��ʾ
��3������[KAl��SO4��2?12H2O]�����ھ�ˮ����������ˮ��Һ�е�����ɫʯ����Һ����Һ��
��4�������ȵ���˿����ˮ���û�ѧ����ʽ��ʾ��
��������1�������������ڸ����¿ɷ�����Ӧ����������������
��2����������Ϊ���������������ǿ�ᡢǿ�Ӧ�����κ�ˮ��
��3������Ϊǿ�������Σ�������ˮ������ԣ���������������
��4������ˮ�����ڸ����·�Ӧ����������������������
��2����������Ϊ���������������ǿ�ᡢǿ�Ӧ�����κ�ˮ��
��3������Ϊǿ�������Σ�������ˮ������ԣ���������������
��4������ˮ�����ڸ����·�Ӧ����������������������
����⣺��1�������������ڸ����¿ɷ�����Ӧ������������������Ӧ�ķ���ʽΪ2Al+Fe2O3
Al203+2Fe���ʴ�Ϊ��2Al+Fe2O3
Al203+2Fe��
��2����������Ϊ���������������ǿ�ᡢǿ�Ӧ�����κ�ˮ����Ӧ�ķ���ʽΪ�ֱ�ΪAl��OH��3+3H+=Al3++3H2O��Al��OH��3+OH-=Al02-+2H2O��
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��Al��OH��3+OH-=Al02-+2H2O��
��3������Ϊǿ�������Σ�������ˮ������ԣ����������������μ���ɫʯ����Һ����Һ�ʺ�ɫ��ˮ�����ӷ���ʽΪAl 3++3H2O?Al��OH��3+3H+��
�ʴ�Ϊ���죻Al 3++3H2O?Al��OH��3+3H+��
��4������ˮ�����ڸ����·�Ӧ������������������������Ӧ�ķ���ʽΪ3Fe+4H2O
Fe3O4+4H2���ʴ�Ϊ��3Fe+4H2O
Fe3O4+4H2��
| ||
| ||
��2����������Ϊ���������������ǿ�ᡢǿ�Ӧ�����κ�ˮ����Ӧ�ķ���ʽΪ�ֱ�ΪAl��OH��3+3H+=Al3++3H2O��Al��OH��3+OH-=Al02-+2H2O��
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��Al��OH��3+OH-=Al02-+2H2O��
��3������Ϊǿ�������Σ�������ˮ������ԣ����������������μ���ɫʯ����Һ����Һ�ʺ�ɫ��ˮ�����ӷ���ʽΪAl 3++3H2O?Al��OH��3+3H+��
�ʴ�Ϊ���죻Al 3++3H2O?Al��OH��3+3H+��
��4������ˮ�����ڸ����·�Ӧ������������������������Ӧ�ķ���ʽΪ3Fe+4H2O
| ||
| ||
���������⿼���Ϊ�ۺϣ��漰��������������ˮ���Լ��������ʣ�Ϊ�߿��������ͺ�Ƶ���㣬ע����ػ���֪ʶ�Ļ��ۣ��ѶȲ���

��ϰ��ϵ�д�
 �żӾ���ϵ�д�
�żӾ���ϵ�д�
�����Ŀ