��Ŀ����
ij�о���ѧϰС��ͬѧ����NaHCO3��KHCO3��ɵ�ij���Ȼ�������ʵ�飬����������ݣ���������ʵ���Ũ����ȣ�
�Լ��㣺
��1������������ᷴӦ�����ӷ���ʽ
��2����������ʵ���Ũ�ȣ�
��3��������������ʵ����ʵ���֮�ȣ�
| 50mL���� | 50mL���� | 50mL���� | |
| m������ | 9.2g | 15.7g | 27.6g |
| V��CO2��������� | 2.24L | 3.36L | 3.36L |
��1������������ᷴӦ�����ӷ���ʽ
H++HCO-3=H2O+CO2��
H++HCO-3=H2O+CO2��
����2����������ʵ���Ũ�ȣ�
��3��������������ʵ����ʵ���֮�ȣ�
��������1��NaHCO3��KHCO3�����ᷴӦ���ʶ���̼������������ӷ�Ӧ���ɶ�����̼��ˮ��
��2��������Ũ����ѡ��������������ݽ��У����Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�꣬�ɱ������ݿ�֪��������ȫ��Ӧ���ɶ�����̼3.36L�����H++HCO-3=H2O+CO2������n��HCl����������c=
���㣻
��3���ɱ������ݿ�֪������9.2g�����ʱ��������ʣ�࣬�������ȫ��Ӧ����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol���������ɶ�����̼�������������֮���з��̼��㣮
��2��������Ũ����ѡ��������������ݽ��У����Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�꣬�ɱ������ݿ�֪��������ȫ��Ӧ���ɶ�����̼3.36L�����H++HCO-3=H2O+CO2������n��HCl����������c=
| n |
| V |
��3���ɱ������ݿ�֪������9.2g�����ʱ��������ʣ�࣬�������ȫ��Ӧ����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol���������ɶ�����̼�������������֮���з��̼��㣮
����⣺��1��NaHCO3��KHCO3�����ᷴӦ���ʶ���̼������������ӷ�Ӧ���ɶ�����̼��ˮ����Ӧ�����ӷ���ʽΪH++HCO-3=H2O+CO2�����ʴ�Ϊ��H++HCO-3=H2O+CO2����
��2���ɱ������ݿ�֪��������ȫ��Ӧ���ɶ�����̼3.36L�����H++HCO-3=H2O+CO2����֪n��HCl��=
=0.15mol���������Ũ��Ϊ
=3mol/L��
�������Ũ��Ϊ3mol/L��
��3����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol��
����xmol+ymol=
��
84g/mol��xmol+100��ymol=9.2g ��
�������̣���ã�x=0.05 y=0.05��
�ʻ������NaHCO3��KHCO3�����ʵ���֮��Ϊ0.05mol��0.05mol=1��1��
�𣺻������NaHCO3��KHCO3�����ʵ���֮��Ϊ1��1��
��2���ɱ������ݿ�֪��������ȫ��Ӧ���ɶ�����̼3.36L�����H++HCO-3=H2O+CO2����֪n��HCl��=
| 3.36L |
| 22.4L/mol |
| 0.15mol |
| 0.05L |
�������Ũ��Ϊ3mol/L��
��3����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol��
����xmol+ymol=
| 2.24L |
| 22.4L/mol |
84g/mol��xmol+100��ymol=9.2g ��
�������̣���ã�x=0.05 y=0.05��
�ʻ������NaHCO3��KHCO3�����ʵ���֮��Ϊ0.05mol��0.05mol=1��1��
�𣺻������NaHCO3��KHCO3�����ʵ���֮��Ϊ1��1��
���������⿼��������йؼ��㣬�Ѷ��еȣ����ݶ�����̼������仯�ж������Ƿ���ȫ��Ӧʽ�ǹؼ����ٸ���������������������ϵ���н�𣬣�3��������ʮ�ֽ��淨����Ϊ��

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ
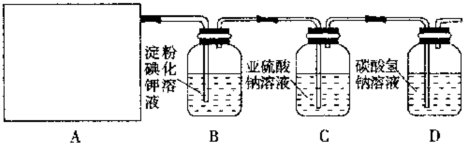
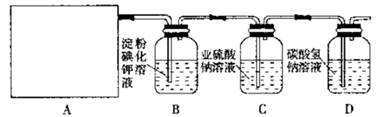
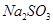 �ѱ�����������ʵ�鲽�裩��
��
�ѱ�����������ʵ�鲽�裩��
��