��Ŀ����
��ѡ�Լ���
a������ʯ��ˮb��Ʒ����Һc�����Ը��������Һd��̼��������Һe������������Һf�����軯����Һg����ˮ
��1����������a�ijɷ֣�������a����ͨ���Լ����Ⱥ�˳��Ϊ��
b��
��2���ڢ۲�ʵ���������Ϊ����Ũ������ȴ�ᾧ��
��3���ڢڲ���H2O2��������
��4����������ͼ��ʽ��ƴ�����C�л��Al��OH��3��������������ͼ������ʾ���ڼ�ͷ�Ϸ����·���������Լ���ʵ�������
��2������Һ����ȡ�����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵķ���������������ȡ���壻
��3����H2O2�������ǰ�Fe2+����ΪFe3+�������������ŵ��Dz��������ʣ�����Ի�������Ⱦ������ҺPH��Ŀ����ʹFe3+��Al3+�γɳ�����
��4��C�к�������������������������������������ǿ������ƫ�����Σ���ƫ��������ͨ�������̼���ɵõ���������������
�ʴ�Ϊ��c��a���ڶ���Ʒ�첻��ɫ�����ҳ���ʯ��ˮ����ǣ�
��2������Һ����ȡ�����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵķ���������������ȡ���壬�����������չ��壬�ʴ�Ϊ�����ˣ�������������
��3����H2O2�������ǽ�Fe2+����ΪFe3+���������������������������ʣ��Ի�������Ⱦ��������ҺPH�����Ӻ�������ȫ����������˵õ���������������������������Һ����ͭ��
�ʴ�Ϊ����Fe2+����ΪFe3+��Fe3+��Al3+��
��4��C�к���������������������������������������������������ǿ������������Һ��������������������������Һ����������������������Һ��Ӧ����ƫ��������Һ������Ӧ�����Һ���й�������������̼ͨ����Һ�У�������̼��ƫ�����Ʒ�Ӧ���������������������˼��ɵõ�����������
���Դ�����C�л��Al��OH��3��������������ͼΪ��
 ��
���ʴ�Ϊ��
 ��
��
 ����ѧ��Ӧ�����ϵ�д�
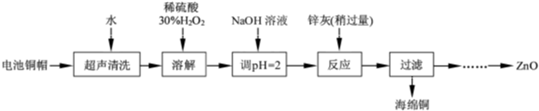
����ѧ��Ӧ�����ϵ�д�[2012�����վ�] ��14�֣���������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɵ�ص�ͭñ(Cu��Zn�ܺ���ԼΪ99%)���� Cu���Ʊ�ZnO�IJ���ʵ��������£�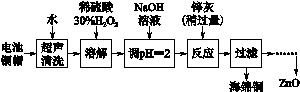
��2����ͭñ�ܽ�ʱ����H2O2��Ŀ����______ _____________________(�û�ѧ����ʽ��ʾ)��
��ͭñ�ܽ���ȫ���轫��Һ�й�����H2O2��ȥ����ȥH2O2�ļ�㷽��____ ___��
��3��Ϊȷ������п��(��Ҫ�ɷ�ΪZn��ZnO������Ϊ������������)������ʵ������ⶨ��ȥH2O2����Һ��Cu2���ĺ�����ʵ�����Ϊ��ȷ��ȡһ������ĺ���Cu2������Һ�ڴ�����ƿ�У�������ˮϡ�ͣ�������ҺpH��3��4�����������KI����Na2S2O3����Һ�ζ����յ㡣���������з�Ӧ�����ӷ���ʽ���£�
2Cu2����4I��===2CuI(��ɫ)����I2
2S2O32����I2===2I����S4O62��
�ٵζ�ѡ�õ�ָʾ��Ϊ________���ζ��յ�۲쵽������____________________��
�����ζ�ǰ��Һ�е�H2O2û�г��������ⶨ��Cu2����������________(�ƫ�ߡ�����ƫ�͡��� ���䡱)��
��֪pH>11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
| | ��ʼ������pH | ������ȫ��pH |
| Fe3�� | 1.1 | 3.2 |
| Fe2�� | 5.8 | 8.8 |
| Zn2�� | 5.9 | 8.9 |
�ɳ�ȥͭ����Һ�Ʊ�ZnO��ʵ�鲽������Ϊ��
��____________________________________________________________��
��__________________________________________________________________��
�۹��ˣ�
��___________________________________________________________________��
�ݹ��ˡ�ϴ�ӡ����
��900 �����ա�
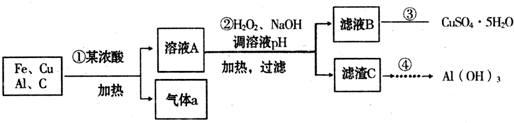
��������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɻ�ͭ(Cu��Zn�Ͻ𣬺���������Fe)�Ʊ���������(CuSO4��5H2O)��������ZnO���Ʊ�����ͼ���£�
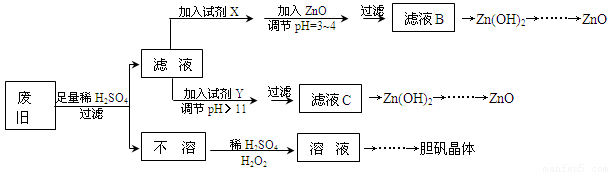
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L��1����)��
| Fe3�� | Fe2�� | Zn2�� |
��ʼ������pH | 1.1 | 5.8 | 5.9 |
������ȫ��pH | 3.0 | 8.8 | 8.9 |
��ش��������⣺
��1���Լ�X������__________����������____________________��
��2������ZnO����pH=3��4��Ŀ����____________________��
��3���ɲ�����������ҺD�Ļ�ѧ����ʽΪ______________________________��
��4������ҺD�Ƶ��������������Ҫ����������______________________________��
��5�������Լ�����ΪY�Լ�����______��
A��ZnOB��NaOHC��Na2CO3D��ZnSO4
������ҺC����μ�������ֱ���������������������______________________________��
��6���ⶨ��������Ĵ���(��������I��������Ӧ������������)��ȷ��ȡ0.5000g��������������ƿ�У�������ˮ�ܽ⣬�ټ������KI����0.1000mol��L��1Na2S2O3����Һ�ζ����յ㣬����Na2S2O3����Һ19.40mL����֪�������ζ������е����ӷ���ʽ���£�
2Cu2����4I�� 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32�� 2I����S4O62��
2I����S4O62��
����������Ĵ���Ϊ_______________��
���ڵζ������о���ҡ��(��Һ���⽦)��ƿ��������õĴ��Ƚ���__________(����ƫ��������ƫ��������������)��
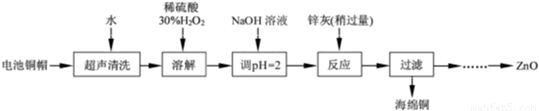
��1����ͭñ�ܽ�ʱ����H2O2 ��Ŀ���� ���û�ѧ����ʽ��ʾ������ͭñ�ܽ���ȫ���轫��Һ�й�����H2O2 ��ȥ����ȥH2O2 �ļ�㷽���� ��
��2��Ϊȷ������п�ң���Ҫ�ɷ�ΪZn��ZnO������Ϊ�����������������ʵ������ⶨ��ȥH2O2 ����Һ��Cu2+�ĺ�����ʵ�����Ϊ��ȷ��ȡһ������ĺ���Cu2+����Һ�ڴ�����ƿ�У�������ˮϡ�ͣ�������ҺpH=3��4�����������KI����Na2S2O3����Һ�ζ����յ㣮���������з�Ӧ�����ӷ���ʽ���£�ҡҡ2Cu2++4I-=2CuI����ɫ����+I2 2S2O32-+I2=2I-+S4O62-
�ٵζ�ѡ�õ�ָʾ��Ϊ ���ζ��յ�۲쵽������Ϊ ��
�����ζ�ǰ��Һ�е�H2O2 û�г��������ⶨ��Cu2+�������� ���ƫ�ߡ�����ƫ�͡����䡱����
��3����֪pH��11 ʱZn��OH��2 ������NaOH��Һ����[Zn��OH��4]2-���±��г��˼������������������������pH��
| ��ʼ������pH | ������ȫ��pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Zn2+ | 5.9 | 8.9 |
�� ���� ���۹��ˣ��� ���ݹ��ˡ�ϴ�ӡ������900�����գ�