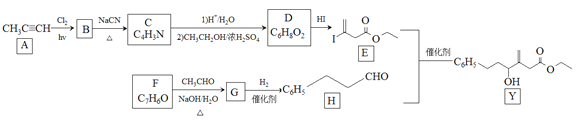
��Ŀ����
����Ŀ��[Cu(NH3)4]SO4��H2O��һ����Ҫ��Ⱦ�ϼ��ϳ�ũҩ�м��塣 ��ش��������⣺
(1)[Cu(NH3)4]2+��ˮ��Һ�е���ɫ��________��
(2)NH3��Nԭ�ӵ��ӻ����������________��
(3)Cu2+��̬��������Ų�ʽΪ_________________��
(4)[Cu(NH3)4]SO4�д��ڵĻ�ѧ�����ͳ��˼��Թ��ۼ��⣬����________��
(5)S��O��N����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_____________��
(6)������ͭͶ�뵽��ˮ��H2O2�Ļ����Һ�У�ͭƬ�ܽ⣬��Һ������ɫ���䷴Ӧ�����ӷ���ʽΪ________��
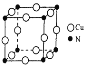
(7)ͭ���Ͻ�ľ����ṹ��ͼ��ʾ���þ����о��������ͭԭ�Ӻ͵�ԭ�Ӻ˼�ľ���Ϊ![]() a pm����þ�����ܶ�Ϊ________ g/cm3( ��NAΪ�����ӵ�����ֵ)��
a pm����þ�����ܶ�Ϊ________ g/cm3( ��NAΪ�����ӵ�����ֵ)��
���𰸡� ����ɫ sp3�ӻ� [Ar]3d9��1s22s22p63s23p63d9 ���Ӽ�����λ�� N>O>S Cu+H2O2+4NH3��H2O=[Cu(NH3)4]2++2OH-+4H2O ![]() ��1030
��1030
��������(1)[Cu(NH3)4]SO4 ��ˮ�д������½�����̣�[Cu(NH3)4]SO4�T[Cu(NH3)4]2+������ɫ���ӣ�+SO42����[Cu(NH3)4]2+=Cu2��+4NH3��(2)NH3������Nԭ�Ӽ۲���ӶԸ���=3+1/2����5-3��1��=4,�ж��ӻ����ͣ���5��ͬһ����Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�صģ���6������ͭͶ�백ˮ��H2O2��Һ�о�����������Ͷ�백ˮ��H2O2�Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ�����й�������Ϊ������������Cu2���γ������ӣ�������ٽ�ʹ��Ӧ���У�����������ԭ��Ӧ�е��ӵ�ʧ�غ�͵���غ���ƽ����7���þ����о��������ͭԭ�Ӻ͵�ԭ�Ӽ�ľ���Ϊ![]() apm�����Ծ����ı߳�Ϊ
apm�����Ծ����ı߳�Ϊ![]() apm���������Ϊ(
apm���������Ϊ(![]() apm��3�����þ�̯�����㾧���к��е�ͭԭ�Ӻ͵�ԭ�Ӹ��������ݦ�=m/V���㣮
apm��3�����þ�̯�����㾧���к��е�ͭԭ�Ӻ͵�ԭ�Ӹ��������ݦ�=m/V���㣮
(1)[Cu(NH3)4]2+��ˮ��Һ�е���ɫ������ɫ��(2)NH3������Nԭ�Ӽ۲���ӶԸ���=3+1/2����5-3��1��=4�Һ���һ���µ��Ӷԣ�����Nԭ�Ӳ���sp3�ӻ���NH3��Nԭ�ӵ��ӻ����������sp3�ӻ���(3)Cu2+��̬��������Ų�ʽΪ[Ar]3d9��1s22s22p63s23p63d9��(4)[Cu(NH3)4]SO4�д��ڵĻ�ѧ�����ͳ��˼��Թ��ۼ��⣬����[Cu(NH3)4]2+������ɫ���ӣ���SO42��֮������Ӽ���N��֮ͭ���ĵ���λ����(5)ͬ��������Ԫ�صĵ�һ�����ܣ�����ԭ����������������������ƣ�����VA����ڵ�VIA��Ԫ�أ�ͬ����Ԫ�أ�����ԭ�����������ӣ���һ��������С����S��O��N����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ��N��O��S��(6)����ͭͶ�백ˮ��H2O2��Һ�о�����������Ͷ�백ˮ��H2O2�Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ�����й�������Ϊ������������Cu2���γ������ӣ�������ٽ�ʹ��Ӧ���У����ӷ���ʽ�ɱ�ʾΪ��Cu+H2O2+4NH3��H2O=[Cu(NH3)4]2++2OH-+4H2O��(7)�ھ����У�Nԭ��λ�ڶ��㣬Cuԭ��λ������е㣬�þ�����Nԭ�Ӹ���=8��1/8=1��Cuԭ�Ӹ���=12��1/4=3������������Ϊ(64��3+14)/NA g�����������Ϊ(![]() apm��3�����=
apm��3�����= =
=![]() ��1030g/cm3��
��1030g/cm3��