��Ŀ����
1������ʵ�顱�ǽ�һ֧��С���Թ�װ����һ�Թ��У�����װ�����ԭ����Ҫ��ֻ������Թܽ��е�ʵ�飮�����������ŵ㣬���������㷺������Ӧ���ڻ�ѧʵ���У��������ʵ��Ϊ����ʵ�顱��ʵ��װ����ͼ��С�Թ��в���մ����ˮ����ͭ��ĩ����֬�ޣ��� �۲�ʵ��װ�ã�����ʵ��ԭ�������ش��������⣺��1������ʵ������У��ܹ۲쵽������Ϊ
���ձ�A��������ð������С�Թ�����֬�ޱ����ɫ���Թ��ڱ���ˮ����������ձ�B���ձ�B�е������������ݲ���������ʯ��ˮ����ǣ�
��2��д��С�Թ��з�����Ӧ�Ļ�ѧ����ʽ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��

���� ��1���ټ���ʱ�����Թ��������������ͣ����Թ۲쵽�ձ�A�������ݲ�����
��̼���������ȷֽ�����̼���ơ�������̼�ͻᣬ��ɫ������ͭ��ˮ��Ӧ������ɫ����ˮ����ͭ��
��̼�����Ʒֽ����ɵĶ�����̼�����ʯ��ˮ��Ӧ����̼��Ƴ�����
��2��̼�����Ʒֽ�����̼���ơ�ˮ��������̼��
��� �⣺��1����ʵ�鿪ʼʱ�����Թ��е������������ͣ��ձ�A�ĵ������л�������ð����
�ʴ�Ϊ��������ð����
��С�Թ��в���մ����ˮ����ͭ��ĩ����֬�ޱ����ɫ���Թ��ڱ���ˮ�������
�ʴ�Ϊ����֬�ޱ����ɫ���Թ��ڱ���ˮ�������
��̼�����Ƽ��ȷֽ����ɶ�����̼���壬������̼�����ʯ��ˮ��Ӧ����̼��Ƴ����������ձ�B�е������������ݲ���������ʯ��ˮ����ǣ�
�ʴ�Ϊ���ձ�B�е������������ݲ���������ʯ��ˮ����ǣ�
��2��̼���������ȷֽ�����̼���ơ�������̼�����ˮ����ӦΪ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
�ʴ�Ϊ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
���� ���⿼��̼���ơ�̼�����Ƶ����ʱȽϣ�Ϊ��Ƶ���㣬����̼�����ƵIJ��ȶ�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | ���� | B�� | �ռ���Һ | C�� | AgNO3��Һ | D�� | KSCN��Һ |
| t/s | 0 | 2 | 4 | 6 | 8 |
| n��Cl2��/mol | 0 | 0.30 | 0.39 | 0.40 | 0.40 |
| A�� | ���������������䣬�����¶ȣ�ƽ��ʱc��Cl2��=0.22 mol•L-1����Ӧ�ġ�H��0 | |
| B�� | ����2 L���ݾ��ȣ������û�������������ܱ��������и÷�Ӧ����ѧƽ�ⳣ������ | |
| C�� | ���������������䣬��ʼ�������г���1.2 molCOCl2��0.60 molCl2��0.60 molCO����Ӧ�ﵽƽ��ǰ�����ʣ�v��������v���棩 | |
| D�� | ���������������䣬��ʼ�������г���1.0 molCl2��0.8 molCO���ﵽƽ��ʱ��Cl2��ת����С��60% |

| A�� | ��NaCl�����У���Na+�����Cl-�γ��������� | |
| B�� | ��CaF2�����У�Ca2+��F-����λ���ֱ���4��8 | |
| C�� | �ڽ��ʯ�����У�̼ԭ����̼̼�������ı�Ϊ1��2 | |
| D�� | ����̬�Ŵط��ӵķ���ʽΪEF��FE |
| A�� | lmol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| B�� | ��ϩ�ͱ�ϩ��ɵ�42 g�����������ԭ�ӵĸ���Ϊ6 NA | |
| C�� | 1 mol Na2O2�����к���������Ϊ4NA | |
| D�� | 2 L0.5 mol•L-1�������Һ�����������������ΪNA |
 $\stackrel{KOH}{��}$H-C��
$\stackrel{KOH}{��}$H-C��
 +H2O��-R��-R�䡢-R���ʾ���ظ���̬����ܲ�ͬ��ԭ�ӻ�ԭ���ţ�
+H2O��-R��-R�䡢-R���ʾ���ظ���̬����ܲ�ͬ��ԭ�ӻ�ԭ���ţ� ����Ӧ�����Ǽӳɷ�Ӧ��
����Ӧ�����Ǽӳɷ�Ӧ��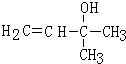 ��
��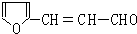 ��
��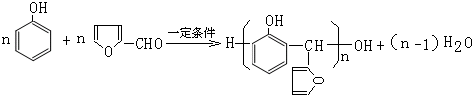 ��
��

 ��
��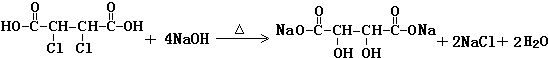 ��
��