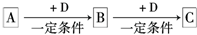��Ŀ����
��10�֣���֪A��B�� C�� D�������ʶ�����һ�ֹ�ͬ��Ԫ�أ��ת���Ĺ�ϵ��ͼ��ʾ��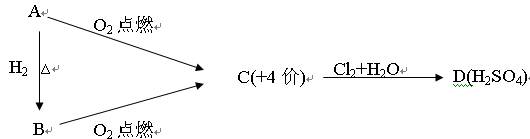
���У�A��һ�ֵ���ɫ�Ĺ�̬�ǽ������ʣ�B��H2S��C��һ����ɫ�д̼�����ζ�����壬����ʹƷ����Һ��ɫ��D��H2SO4��
��1������������Ϣ�ƶϣ�A�� C�� ���ѧʽ��
��2����A�� B�� C�� D������������ѡһ�֣�������������ͬԪ�صļ�̬��Ԥ������ʾ��������Ի�ԭ�ԣ������ʵ����֤��
�ҵ�Ԥ�⣺
��Ҫ������
���ܵ�����
��ѧ����ʽ��
��1��S SO2
��2���ҵ�Ԥ�⣺S���л�ԭ��
��Ҫ������ȡ����S����ȼ�ճ��е�ȼ������װO2�ļ���ƿ��
���ܵ�������ȼ�ղ���������������ɫ����
��ѧ����ʽ��S+O2 SO2��������������ɣ�
SO2��������������ɣ�
����

��ϰ��ϵ�д�
�����Ŀ
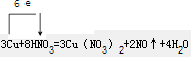
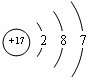
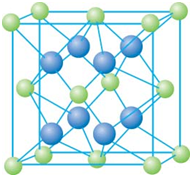 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�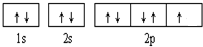
 A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮���ת����ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�A��I�����ֳ����Ľ������ʣ�H�ڳ�������һ�ֳ����ķǽ�����̬���ʣ�
A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮���ת����ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�A��I�����ֳ����Ľ������ʣ�H�ڳ�������һ�ֳ����ķǽ�����̬���ʣ�