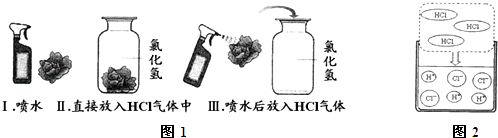
��Ŀ����
����Ŀ������ͭ����Ҫ�Ĺ���Ԫ�أ��ڹ�ũҵ����������Ҫ�����á��ش��������⣺
��1����Ԫ�������ڱ��е�λ��__________________________��
��2�������ۡ�ͭ�ۡ�FeCl3��FeCl2��CuCl2��Һ�����ij�����г�ַ�Ӧ��
���ж���������£���Һ�д��ڵĽ������Ӻͽ������ʡ�
����������ʣ�࣬�������в������У�____________��
���������ڻ����д�����Fe3+���������л��У�_______________ ��
���������ڻ��н϶��Cu2+���൱����Cu���������ڲ�������_______________��
��3��ij��Һ�к���Fe3+��Br���������֤����Fe2+________________________________����ʹ�õ��Լ��У�KSCN��Һ��AgNO3��Һ�����Ƶ���ˮ��ϡ���ᡢNaOH��Һ������KMnO4��Һ��˫��ˮ��
��4��ͭ��ұ������֮һ����ͭ����Ҫ�ɷ���2CuCO3��Cu(OH)2�����뽹̿һ�����ʱ����������ͭ��CO2����ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
���𰸡� ��������VIII�� Fe3+��Cu2 Fe2+��Cu2+ Fe3+�������� ȡ������Һ�����������������Һ�����ˣ�����Һ�м������Ը�����أ���ɫ 2��2CuCO3��Cu(OH)2��+3C![]() 6Cu+7CO2��+2H2O
6Cu+7CO2��+2H2O
��������(1)����26��Ԫ�أ�λ�����ڱ��е������ڵڢ��壬�ʴ�Ϊ���������ڵڢ��壻
(2)�����ԣ�FeCl3��CuCl2��FeCl2����ԭ��Fe��Cu��
�ٷ�Ӧ������ʣ�࣬����Fe+2FeCl3=3FeCl2��Fe+CuCl2=Cu+FeCl2��Fe3+��Cu2+�����ܴ��ڣ��ʴ�Ϊ��Fe3+��Cu2+��
���������ڻ����д�����Fe3+�������Ӿ��������ԣ��ܹ��ܽ�����ͭ����������һ������Fe2+��Cu2+���ʴ�Ϊ��Fe2+��Cu2+��
���������ڻ��н϶��Cu2+���൱����Cu�������Ӿ��������ԣ��ܹ��ܽ�ͭ���������ڲ�������Fe3+��Cu2+�ܹ��ܽ�������������ڲ�������Fe���ʴ�Ϊ��Fe3+�������ʣ�
(3)�������Ӿ��л�ԭ�ԣ���������Ҳ�л�ԭ�ԣ�����ʵ�ּ����������������ӳ�ȥ�����ø�����ؼ��飬ʵ�鷽��Ϊ��ȡ������Һ�����������������Һ�����ˣ�����Һ�м������Ը�����أ���ɫ���������֤����Fe2+���ʴ�Ϊ��ȡ������Һ�����������������Һ�����ˣ�����Һ�м������Ը�����أ���ɫ��
(4)���ݷ�Ӧ�����������д����ʽΪ2[2CuCO3Cu(OH)2]+3C![]() 6Cu+7CO2��+2H2O���ʴ�Ϊ��2[2CuCO3Cu(OH)2]+3C
6Cu+7CO2��+2H2O���ʴ�Ϊ��2[2CuCO3Cu(OH)2]+3C![]() 6Cu+7CO2��+2H2O��
6Cu+7CO2��+2H2O��

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�����Ŀ��ϩ���ļ�������Ӧʵ��֮һ��CH3CH=CH2��CO��H2�ڴ��������¿ɺϳ�����ȩ��CH3 CH2 CH2 CHO�����йط�Ӧ���£�
��CH3CH=CH2��g��+CO ��g��+H2��g��![]() CH3CH2CH2CHO��g�� ��H1
CH3CH2CH2CHO��g�� ��H1
����Ӧ�У�
��CH3CH=CH2��g��+ CO ��g��+ H2��g��![]() ��CH3��2CHCHO��g�� ��H2
��CH3��2CHCHO��g�� ��H2
��CH3CH=CH2��g��+ H2��g�� �� CH3 CH2 CH3��g�� ��H3
�ش��������⣺
��1�������Ǹ���Ӧ�������ɫ��ѧ�Ƕȿ��ǣ�ϩ����������Ӧ����Ҫ�ŵ���_______________________________��
��2����֪���м������ݣ�
��ѧ�� | C-H | C-C | C=C | H-H |
����/KJ��mol��1 | 413.3 | 347.7 | 615.0 | 436.0 |
��ӦCH3CH=CH2��g��+ H2��g�� �� CH3 CH2 CH3��g������H3=_______________��
��3���ں����ܱ������г���1 mol��ϩ������ij�ִ����ڼ��������½��з�Ӧ������¶����ϩ����ƽ��ת���ʼ������칹������ʵ���֮�ȵĹ�ϵ����ͼ��ʾ��
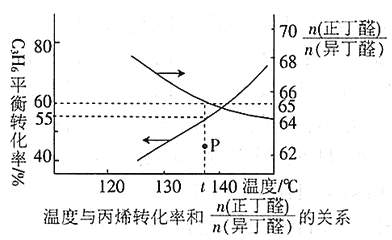
�ٱ�ϩ�ϳ�����ȩ��CH3 CH2 CH2 CHO�����춡ȩ����CH3��2CHCHO���ķ�Ӧ����______________(��������������������)��Ӧ��
��t��ʱ����Ӧ��CH3��2CHCHO��g��![]() CH3 CH2 CH2 CHO��g����ƽ�ⳣ��K =____________________��������������Ӧ�٢ڣ���Ӧ�ﵽƽ��ʱ������������ȩ�����ʵ���Ϊ_______��
CH3 CH2 CH2 CHO��g����ƽ�ⳣ��K =____________________��������������Ӧ�٢ڣ���Ӧ�ﵽƽ��ʱ������������ȩ�����ʵ���Ϊ_______��
��t��ʱ��ͼ��P��ķ�Ӧ����v������_______v���棩(����<�� ��>������=��)��
��Ҫ�������ȩ�IJ��ʳ����ú��ʵ��¶��⣬����_______________________��