��Ŀ����
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������£�N2H4���ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�á�
��.��1��N2H4�е�Nԭ�������ﵽ8�����ȶ��ṹ��д��N2H4�Ľṹʽ_____________��
��2��NH3��NaClO��Ӧ�ɵõ��£�N2H4�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������һ�ָ���ȼ�ϣ��йػ�ѧ��Ӧ�������仯����ͼ��ʾ��д����ȼ�յ��Ȼ�ѧ����ʽ ��
��.���ĺϳ�������Ҫ�Ļ�������֮һ����֪��
N2��g����3H2��g�� 2NH3��g����H����92.4 kJ��mol��1
2NH3��g����H����92.4 kJ��mol��1
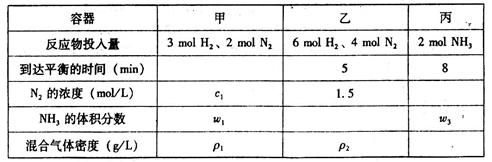
��3�������Ϊ2L���ܱ������У�����ͬ���¶��£�ʹ����ͬ�Ĵ����ϳɰ���ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ��
�Իش�
��1�����и�����˵���÷�Ӧ�Ѵﵽƽ��״̬����______________����д�����ĸ����
a��������N2��H2��NH3��Ũ��֮��Ϊ1�U3�U2
b��v��N2������3v��H2����
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
��2�������ϱ����ݣ����й�ϵ��ȷ����_________����д�����ĸ����
��3���������з�Ӧ�ӿ�ʼ����ƽ��ƽ������Ϊv(H2)= _____________��
III.ֱ�ӹ���ʽ����ȼ�ϵ�صĵ�ط�Ӧʽ��4NH3+3O2=2N2+6H2O���������Һһ��ʹ��KOH��Һ�����缫��Ӧʽ��__________ ��
��.��1��N2H4�е�Nԭ�������ﵽ8�����ȶ��ṹ��д��N2H4�Ľṹʽ_____________��

��2��NH3��NaClO��Ӧ�ɵõ��£�N2H4�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������һ�ָ���ȼ�ϣ��йػ�ѧ��Ӧ�������仯����ͼ��ʾ��д����ȼ�յ��Ȼ�ѧ����ʽ ��
��.���ĺϳ�������Ҫ�Ļ�������֮һ����֪��
N2��g����3H2��g��
 2NH3��g����H����92.4 kJ��mol��1
2NH3��g����H����92.4 kJ��mol��1��3�������Ϊ2L���ܱ������У�����ͬ���¶��£�ʹ����ͬ�Ĵ����ϳɰ���ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ��

�Իش�
��1�����и�����˵���÷�Ӧ�Ѵﵽƽ��״̬����______________����д�����ĸ����
a��������N2��H2��NH3��Ũ��֮��Ϊ1�U3�U2
b��v��N2������3v��H2����
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
��2�������ϱ����ݣ����й�ϵ��ȷ����_________����д�����ĸ����
| A��2c1��1.5mol��L-1 | B��w3=w1 | C��2��1=��2 | D��K��= K��= K�� |
III.ֱ�ӹ���ʽ����ȼ�ϵ�صĵ�ط�Ӧʽ��4NH3+3O2=2N2+6H2O���������Һһ��ʹ��KOH��Һ�����缫��Ӧʽ��__________ ��
��1��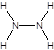 ��1�֣�
��1�֣�
��2�� 2NH3+NaClO��N2 H4+NaCl+H2O��2�֣�
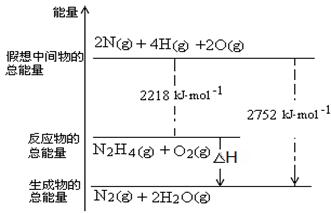
��3��N2 H4(g) + O2(g) = N2(g) + 2H2O(g ) ��H����534 kJ��mol��1��2�֣�
��1��C��2�֣�
��2��A C D��3�֣�
��3��0.3 mol��L-1��min-1��2�֣�
III��2NH3 + 6OH- + 6e- = N2 + 6H2O��2�֣�
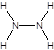 ��1�֣�
��1�֣���2�� 2NH3+NaClO��N2 H4+NaCl+H2O��2�֣�
��3��N2 H4(g) + O2(g) = N2(g) + 2H2O(g ) ��H����534 kJ��mol��1��2�֣�
��1��C��2�֣�
��2��A C D��3�֣�
��3��0.3 mol��L-1��min-1��2�֣�
III��2NH3 + 6OH- + 6e- = N2 + 6H2O��2�֣�
�����������1��N2H4�е�Nԭ�ӿɴﵽ8���ӵ��ȶ��ṹ����ԭ�������3�������γ����Թ��õ��Ӷԣ���δ�ɼ���һ�Ե����γ�8�����ȶ��ṹ��ÿ����ԭ�Ӻ�������ԭ���γɹ��ۼ�����ԭ�Ӽ��γ�һ�����ۼ����ṹʽΪ��
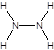 ���ʴ�Ϊ��
���ʴ�Ϊ��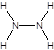 ��
����NH3��NaClO��Ӧ�ɵõ��£�N2H4�����÷�Ӧ�Ļ�ѧ����ʽΪ��2NH3+NaClO��N2 H4+NaCl+H2O���𰸣�2NH3+NaClO��N2 H4+NaCl+H2O���Ǵ�ͼ�ж�������H=22218kJ��mol��1-2752kJ��mol��1=��534 kJ��mol��1��������ȼ��������̬ˮ���Ȼ�ѧ����ʽΪ��N2 H4(g) + O2(g) = N2(g) + 2H2O(g ) ��H����534 kJ��mol��1���𰸣�N2 H4(g) + O2(g) = N2(g) + 2H2O(g ) ��H����534 kJ��mol��1��
��A��Ӧ������֮�����жϣ���Ӧ�����а��ձ������з�Ӧ����A�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��B����������֮�ȵ���ϵ��֮�ȣ�������������Ӧ���ʣ���ij�����ʵ�����Ӧ���ʺ��淴Ӧ��������DZ�����Ӧ�ﵽƽ�⣬���Ե�3v��N2����=v��H2���� ʱ��Ӧ�ﵽƽ�⣬��B�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��C����������ѹǿ���ֲ��䣬˵����Ӧ�ﵽƽ�⣬��C���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����Ӧ��ϵ�������غ㣬���һ�����ʻ��������ܶȲ��䣬���Ի��������ܶȱ��ֲ��䣬����˵����Ӧ�ﵽƽ�⣬��D�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬���𰸣�C��
��A����ȷ������1.5L�������������ҵ�Чƽ�⣬Ȼ��Ŵ�3L��ƽ��������N2�ķ����淽���ƶ�������2c1>1.5 mol��L �D1; B������ȷ������Ч��3molH2,1molN2����ȷ����ƽ�⣬�����1molN2��ƽ�������ƶ���C����ȷ����Ӧ��ϵ�������غ㣬���һ�����������������������ҵ�2�������Ի��������ܶȣ�2��1=��2��D����ȷ���¶Ȳ��䣬ƽ�ⳣ�����䣬K��= K��= K�����𰸣�A C D��
��v(H2)= 3v(N2)=
 ���𰸣�0.3 mol��L-1��min-1��
���𰸣�0.3 mol��L-1��min-1��III.NH3����ԭ��������1molN2ʧȥ6mol���ӣ���OH�D����ʹ����ʽ���ߵ���غ㣬������Ӧ��2NH3 + 6OH- + 6e- = N2 + 6H2O���𰸣�2NH3 + 6OH- + 6e- = N2 + 6H2O

��ϰ��ϵ�д�
�����Ŀ
 2AB3��g���Ħ�H��0
2AB3��g���Ħ�H��0 4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%��
4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%�� CH3OH(g) ��H = ��90.8kJ/mol��
CH3OH(g) ��H = ��90.8kJ/mol��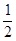 O2(g)=H2O(g) ��H = ��241.8kJ/mol��
O2(g)=H2O(g) ��H = ��241.8kJ/mol�� H2(g)+ CO2(g) ��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1������ʼʱc(CO)=1mol?L-1��c(H2O)=2mol?L��1���Իش��������⣺
H2(g)+ CO2(g) ��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1������ʼʱc(CO)=1mol?L-1��c(H2O)=2mol?L��1���Իش��������⣺
 CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ �� CO (g)+2H2O (g) +519KJ����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
CO (g)+2H2O (g) +519KJ����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��