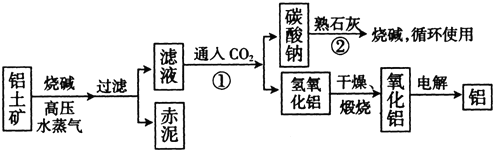
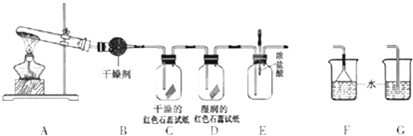
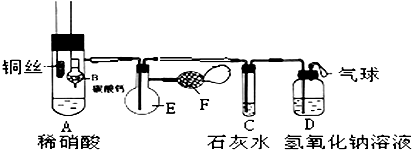
��Ŀ����
��ӦCaCO3=CaO��CO2���ڲ�ͬ�¶��£�CO2��ƽ��ѹǿ���±���
�¶ȣ����������� 550���������������� 650���������������� 750���������������� 850���������������� 897
ѹǿ��Pa�������� 5.32��10�������� 9.17��102�������� 8.37��103�������� 4.94��104�������� 1.01��105
�밴Ҫ����գ�
��1��������ԭCaCO3�ֽ�ƽ����ϵ���¶ȡ�ͬʱ����Ӧ������ѹ����ԭƽ��________��
( )
A. �����ƶ�
B. �����ƶ�
C. ���ƶ�
D. ��ȷ��ƽ���ƶ�����
��2����һ������粻�ܽ����Ƚ������ܱ������У�������CaCO3��850��ʱ�����˷� ��ƽ�⡣�������������ݻ�����Ϊԭ����2���������´ﵽƽ��ʱ�����ڵ��¶Ƚ� ________��CO2��ƽ��ѹǿ��________4.94��104Pa��������________��
�𰸣�
������
������
��1��D ��2������ С�� �������ʹCO2ѹǿ��С��ƽ�������ƶ�����Ϊ��Ӧ���ȣ���ʹ��ϵ�¶Ƚ��ͣ�����ƽ��ʱ��CO2ѹǿ��С��ԭƽ���ѹǿ
|

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ