��Ŀ����
ˮ�ĵ���ƽ��������ͼ��ʾ��
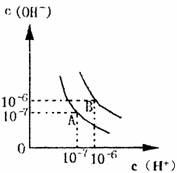
��1������A���ʾ25��ˮ�ڵ���ƽ��ʱ������Ũ�ȣ����¶�������100��ʱ��ˮ�ĵ���ƽ��״̬��B�㣬���ʱˮ�����ӻ��� ���ӵ� �����ˮ�����ӻ������ԭ���� ___________________
��
��2����֪��25��ʱ��0.1mol/L��H2R��Һ��0.7<pH<1����֪1g2=0.3������H2R��ˮ��Һ�еĵ��뷽��ʽΪ
��3��100��ʱ����pH=9��NaOH��Һ��pH=4��������Һ��ϣ���������ҺpH=7����NaOH��Һ��������Һ�������Ϊ ��
��4��100��ʱ����10�����ijǿ����Һ��1�����ijǿ����Һ��Ϻ���Һ�����ԣ�����֮ǰǿ����Һ��pH��ǿ����Һ��pH֮��Ӧ����Ĺ�ϵ�� ____________________________
_____ ��
��1��10�D14 10�D12 �¶����ߣ�ˮ�ĵ���̶�������Һ�е�H+��OH�DŨ������Kw����
��2��H2R=H++HR�D HR�D ![]() H++R2�D
H++R2�D
��3��1:9
��4��pH��+pH��=13

 ˮ�ĵ���ƽ��������ͼ��ʾ��
ˮ�ĵ���ƽ��������ͼ��ʾ�� ��2006?�ɶ�ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ��
��2006?�ɶ�ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ�� ��2012?����һģ����1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��Cʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�100��ʱ1mol?L-1 ��NaOH��Һ�У���ˮ�������c��H+��=
��2012?����һģ����1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��Cʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�100��ʱ1mol?L-1 ��NaOH��Һ�У���ˮ�������c��H+��= ��2011?��̨ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ��
��2011?��̨ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ��