��Ŀ����
.(10��)80��ʱ����0.40mol��N2O4�������2L�Ѿ���յĹ̶��ݻ����ܱ������У��������·�Ӧ��N2O4(g) 2NO2(g) ��H>0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2(g) ��H>0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
| ʱ��(s) n(mol) | 0 | 20 | 40 | 60 | 80 | 100 |
| n(N2O4) | 0.40 | a | 0.20 | c | d | e |
| n(NO2) | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
�ڼ�����80��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��
�۷�Ӧ������100s��Ӧ�������¶Ƚ��ͣ�����������ɫ(���dz������������䡱) ��
��Ҫ����÷�Ӧ��Kֵ���ɲ�ȡ�Ĵ�ʩ��(�����) ��
A.����N2O4����ʼŨ�� B.����������ͨ��NO2
C.ʹ�ø�Ч���� D.�����¶�
����ͬ�����£������0.40mol��N2O4�������2L�Ѿ���յĹ̶��ݻ��ľ����ܱ������У����մﵽƽ���n(NO2) 0.60 mol(�> ������<����=��)��
(1)��0.002 mol/(L��s)����1.8 mol/L�� �۱�dz�� �� D �� <
����

��10��)��ش��������⣺
��1�����¡������£���֪2NO+O2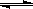 2NO2 ��H<0�����÷�Ӧ����ƽ��״̬ʱ�����д�ʩ�����������NO��ƽ��ת���ʵ��� ������ĸ����
2NO2 ��H<0�����÷�Ӧ����ƽ��״̬ʱ�����д�ʩ�����������NO��ƽ��ת���ʵ��� ������ĸ����
| A�������¶� | B�������¶� | C������O2 | D����Сѹǿ |
��2������l.00 molO2��2.00 mol NO��ϳ����ݻ�Ϊ2 L���ܱ������У�3���Ӻ�Ӧ�ﵽƽ�⡣ƽ��������������ʵ���Ϊ2.55mol����O2��ʾ�Ļ�ѧ��Ӧ����Ϊ mol?L-1?min-1��
��3���ݻ���Ϊ2 L���ĸ��ܱ������о������ţ�2���еķ�Ӧ��ij�¶��£����������ʵ�����mol�������淴Ӧ���ʹ�ϵ���±���ʾ��
| ������� | n(O2) | n(NO) | n (NO2) | v����v���Ĺ�ϵ |
| �� | 0.20 | 0.10 | 0.20 | v����v�� |
| �� | 0.20 | 0.40 | 1.00 | ��v��___v��? |
| �� | 0.60 | 1.20 | 0.80 | ��v��___v��? |
��д���пո� �� �� (��>�� <��
.(10��)80��ʱ����0.40mol��N2O4�������2L�Ѿ���յĹ̶��ݻ����ܱ������У��������·�Ӧ��N2O4(g) 2NO2(g) ��H>0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2(g) ��H>0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
|
ʱ��(s) n(mol) |
0 |
20 |
40 |
60 |
80 |
100 |
|
n(N2O4) |
0.40 |
a |
0.20 |
c |
d |
e |
|
n(NO2) |
0.00 |
0.24 |
b |
0.52 |
0.60 |
0.60 |
�ټ���20s��40s����N2O4��ʾ��ƽ����Ӧ����Ϊ ��
�ڼ�����80��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��
�۷�Ӧ������100s��Ӧ�������¶Ƚ��ͣ�����������ɫ(���dz������������䡱) ��
��Ҫ����÷�Ӧ��Kֵ���ɲ�ȡ�Ĵ�ʩ��(�����) ��
A.����N2O4����ʼŨ�� B.����������ͨ��NO2
C.ʹ�ø�Ч���� D.�����¶�
����ͬ�����£������0.40mol��N2O4�������2L�Ѿ���յĹ̶��ݻ��ľ����ܱ������У����մﵽƽ���n(NO2) 0.60 mol(�> ������<����=��)��
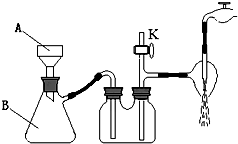 ������ع㷺���ڻ�����ȵ��ҵ��ʵ������ȡ������ص�ԭ�����ս̰桶ʵ�黯ѧ���С�����ؾ�����Ʊ�����ͬ������Ϊ����ȡһ��������KCl��NaClO4�����ܽ⣬����ȴ�ᾧ�����ˡ��˳�����������ˮ���ϴ�Ӽ���ո���õ���
������ع㷺���ڻ�����ȵ��ҵ��ʵ������ȡ������ص�ԭ�����ս̰桶ʵ�黯ѧ���С�����ؾ�����Ʊ�����ͬ������Ϊ����ȡһ��������KCl��NaClO4�����ܽ⣬����ȴ�ᾧ�����ˡ��˳�����������ˮ���ϴ�Ӽ���ո���õ��� ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺