��Ŀ����
����A��G������ѧ�������������֮������ͼ��ת����ϵ�����ַ�Ӧ�����������ȥ������֪BΪһ�����壬E��F��G���ֻ������о�����ͬһ��Ԫ��M���Ҽ�̬������ͬ����ش��������⡣
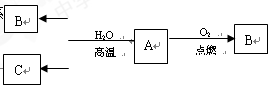
��1��MԪ��Ϊ____________��
��2��B��_________��_________��д�����ּ������ǵķ���________��____________��
��3����A��G��ӦҲ����B���ҷ�Ӧ��n��A��:n��G��=1:2�����ʱ�÷�Ӧ�����ӷ���ʽ��________________________��
��4����B��F��һ�������·�Ӧ����һ�ֻ������һ�ֵ��ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ________________________��

��1��MԪ��Ϊ____________��
��2��B��_________��_________��д�����ּ������ǵķ���________��____________��
��3����A��G��ӦҲ����B���ҷ�Ӧ��n��A��:n��G��=1:2�����ʱ�÷�Ӧ�����ӷ���ʽ��________________________��
��4����B��F��һ�������·�Ӧ����һ�ֻ������һ�ֵ��ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
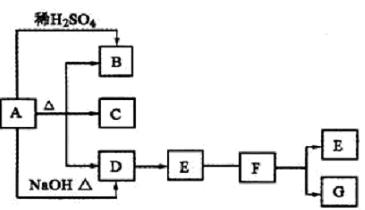
��1��N��3�֣�
��2��SO2�� CO2��ÿ��1�֣���2�֣�����Bͨ��Ʒ����Һ������Һ��ɫ����ΪSO2���粻��ɫ����ΪCO2����Bͨ������KMnO4��Һ�У�����ɫ����ΪSO2���粻��ɫ����ΪCO2����ÿ��2�֣���4�֣�������������Ҳ���֣�
��3��2H+��CO2-3 =CO2����H2O��3�֣��������SO2�ķ�Ӧ���÷֣�
��4��2NO2��4SO2=N2��4SO3��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
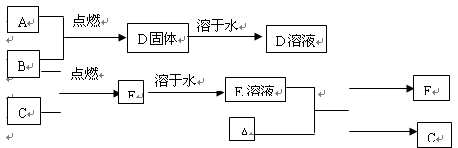
 ��1��д��G�ĵ���ʽ ��
��1��д��G�ĵ���ʽ ��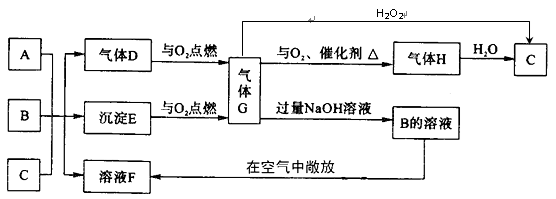
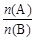 Ӧ����Ĺ�ϵ�� ��
Ӧ����Ĺ�ϵ�� ��
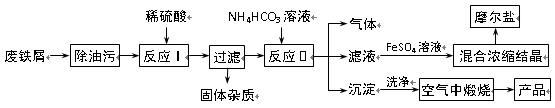 ��֪��FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3������Һ��
��֪��FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3������Һ��